Abstract
Leaf extract of Rubus suavissimus. S. Lee (Rosaceae), a perennial woody shrub indigenous to southern China, was tested for its biological activity in the regulation of transcription factor NF-κ.B (nuclear factor kappa B) A NF-κ.B luciferase reporter cell line was used in this study. The crude extract inhibited NF-κ.B at 100–1000 µg/ml concentrations with minor cytotoxicity. This finding prompted two more rounds of fractionation toward separating the active fraction from the cytotoxic fraction. Five fractions were obtained in the first round of fractionation, of which RUS-C20 was found to be the most potent in inhibiting NF-κ.B without signs of cytotoxicity up to 1000 µg/ml concentration. Fraction RUS-C100 showed little inhibition to NF-κ.B and apparent cytotoxicity. Further fractionation of RUS-C20, using Sephadex LH-20 chromatography, resulted in three subfractions that were distinct in chemical constituents as determined by their retention times and UV absorption spectra of each component. NF-κ.B reporter assay indicated that one of the three subfractions of RUS-C20 retained the inhibitory activity, whereas the other two subfractions were inactive. Bioassay-guided fractionation led to a successful separation of efficacy from cytotoxicity and identification of active components. This warrants further isolation for a purified single compound, which can be used as the chemical marker to standardize the extract for inhibition of NF-κ.B.
Introduction
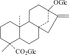
Rubus suavissimus. S. Lee (Rosacene) is a perennial shrub in the rose family. It is widely distributed in southwestern China but flourishes in the Guangxi Zhuang Autonomous Region. R. Suavissimus. leaf has long been used in southern China as a beverage leaf tea due to its sweet taste, thus its Chinese name tiancha, or “sweet tea.” The sweet taste is due to the presence of diterpene glucosides in the leaf, one of which is rubusoside (), reportedly reaching a concentration of over 5% (w/w) (Tanaka et al., Citation1981; Seto et al., Citation1984). Rubusoside is approximately 110-times sweeter than sucrose at the concentration of 0.0025% but has a slightly bitter aftertaste. There were other diterpene glucosides found in the leaf, such as suavioside A (Hirono et al., Citation1990; Zhou et al., Citation1992) and suaviosides B, C1, D2, F, G, H, I, and J (Ohtani et al., Citation1992). Further chemical analyses of the leaves of 39 other Rubus. spp. revealed that the presence of diterpene glycosides is limited to the leaves of R. suavissimus. and R. chingii., whereas glucosyl 19α-hydroxyursana-type triterpenes are more common as constituents in the leaf of Rubus. spp. (Gao et al., Citation1985; Kitahata et al., Citation1989).
In southern China, especially Guangxi, the leaf of R. suavissimus. is used not only as a beverage tea and food additive but also as a folk herbal medicine. It is used to “nourish the kidneys,” lower blood pressure, and relieve diabetic symptoms (Huang & Jiang, Citation2002). The leaf has also been used as an herbal ingredient in decoctions taken for “clearing the internal heat,” “relieving the stress of lungs,” reducing the secretion of phlegm, and relieving coughs (Deng, Citation1997). Recent studies have indicated anti-inflammatory effects and allergy relief (Nakahara et al., Citation1996; Kotaro, Citation1997; Nakahara, Citation1998; Ono, Citation2002). Despite the presumable safe use of R. suavissimus. as food and medicine, published reports of its medical use are scarce, a common phenomena seen in many of the herbs being used in human populations.
The involvement of NF-κ.B in the expression of numerous cytokines and adhesion molecules has made it the most extensively studied transcription factor in the immune system. NF-κ.B activities have been associated with many diseases including diabetes, athersclerosis, cancer, arthritis, and inflammation (Baldwin, Citation2001). We hypothesized that the leaf extract of R. suavissimus. might act through inhibition of NF-κ.B activity for its therapeutic efficacy. NF-κ.B is a master transcription factor in the regulation of inflammation. Our studies suggest that an increase in NF-κ.B activity contributes to the pathogenesis of type 2 diabetes (Gao et al., Citation2002Citation2004). Inhibition of NF-κ.B leads to reduction in diabetes risk (Gao et al., Citation2003Citation2004). In this study, we tested our hypothesis using a NF-κ.B reporter assay.
In addition to testing the hypothesis, we attempted to identify the bioactive component in the leaf extract of R. suavissimus.. Our approach was to identify the bioactivity first and then use this activity to guide fractionation and isolation. Such an approach is illustrated in this paper.
Materials and Methods
Plant material
The leaves of R. suavissimus. were collected from Jinxiu, Guangxi, People's Republic of China, in June 2000 and identified by researchers at the Department of Resources and Environment Sciences, Guangxi Normal University, China. The collected leaves were air-dried and stored at room temperature until extraction.
Crude extraction
Dried leaf material (1 kg) of Rubus suavissimus. was soaked in 20 l of distilled water (1:20 w/v) for 1 h and brought to boil for 30 min twice. The water extract was subsequently filtered using cheesecloth and finally spray-dried to obtain 310 g of crude extract powder, RUS-C.
Fractionation
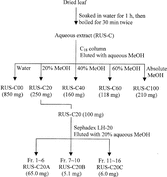
RUS-C (2 g) was reconstituted using 20 ml of deionized water. The aqueous extract was loaded onto a 40 g C18 column (Varian Analytical Instrument, Walnut Creek, CA, USA) and eluted with 150 ml of water, 20% aqueous MeOH, 40% aqueous MeOH, 60% aqueous MeOH, and absolute MeOH in sequence to obtain the extract fraction eluates (). These eluates were evaporated to remove MeOH under reduced pressure and subsequently freeze-dried to afford five fractions as RUS-00 (850 mg), RUS-20 (250 mg), RUS-40 (160 mg), RUS-60 (118 mg), and RUS-100 (210 mg), respectively. Then, 100 mg of RUS-20 was submitted to 50 g Sephadex LH-20 chromatography (Sigma Chemical Company, St. Louis, MO, USA) using 20% aqueous MeOH to elute. A total of 16 fractions, each 10 ml, were collected as a result. The collected fractions were monitored on a high-performance liquid chromatograph in order to determine their chromatographic similarities (described below). Fractions that were similar chromatographically were combined. The combined fractions were evaporated under reduced pressure and subsequently freeze-dried into an extract powder.
Figure 2 Diagram depicting the extraction and fractionation procedures of R. suavissimus. leaf material.
The high-performance liquid chromatography (HPLC) system (Beckman Instruments, Fullerton, CA, USA) consisted of a Model 125 pump, Model 168 photodiode array detector, and Model 502 autosampler. The separation was performed on an ODS (C18) column (150 × 4.6 mm, 5 µm, Alltech Associates, Inc., Deerfield, IL, USA). Chromatographic fingerprints of the crude extract and fractions were developed. The mobile phase was 0.15% aqueous acetic acid/MeOH (9:1 v/v) with a flow rate of 1.0 ml/min. The monitoring wavelength was set at 260 nm in real-time. However, the UV absorption spectrum for each major peak was obtained with the UV diode array detector ranging from 190 nm to 440 nm.
NF-κ.B reported assay
NF-κ.B luciferase reporter DNA plasmid, in which expression of the reporter luciferase is under the control of five NF-κ.B binding sites, was used to transfect mouse macrophage cell line RAW264.7 to make a stable NF-κ.B reporter cell as described (Zhang et al., Citation2001). NF-κ.B activity was induced by addition of lipopolysaccharide (LPS) in the cell culture medium. The enzyme activity of luciferase was used to determine NF-κ.B activity. To examine the bioactivity of R. suavissimus. leaf, the extract and fractions were dissolved in PBS and added to the cell culture medium 30 min before LPS. After 6 h of incubation with the herbal extract, the reporter cells were harvested and tested for NF-κ.B activity. A decrease in the LPS-induced NF-κ.B activity is an indication of NF-κ.B inhibition; an increase in activity is an indication of NF-κ.B activation. Cytotoxicity of a compound is determined by examining the cell morphology under a microscope.
Results
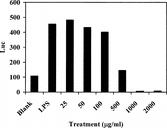
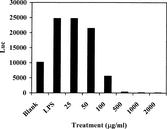
The crude extract RUS-C was the water-soluble part of the raw leaf and accounted for 31% w/w. RUS-C was tested in the NF-κ.B reporter assay and was found to inhibit NF-κ.B at 500 µg/ml in the cell culture medium () with some cytotoxicity (data not shown). In order to separate the inhibitory activity from toxicity, the crude extract was subjected to two rounds of fractionation.
Figure 3 Inhibition of LPS-induced NF-κ.B luciferase (Luc) activity by RUS-C after 6 h of co-culture (n = 3).
The first round of fractionation from RUS-C was done in a chromatographic C18 column. Five fractions (RUS-C00, RUS-C20, RUS-C40, RUS-C60, and RUS-C100) were obtained. Analyses of their chromatographic fingerprints indicated differences in chemical compositions of the fractions despite some overlapping constituents suggesting a successful initial fractionation. The RUS-C extract contained four major groups of compounds that were eluted between the retention times of 0 and 20 min as distinguished by their UV absorption spectra (190 to 440 nm). Fraction RUS-C20 contained three major and distinctive compounds that were eluted around 2.9 to 3.7, 4.7 to 5.5, and 17.4 to 18.2 min. Fractions RUS-C00 and RUS-C40 contained similar compounds but in reduced quantity. Fraction RUS-C100 contained primarily two compounds that also appeared in both RUS-C20 and RUS-C60 fractions. Fraction RUS-C60 contained similar compounds present in fraction RUS-C20 and fraction RUS-C100 but in far lesser amounts.
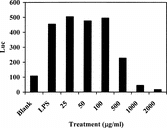
All five fractions obtained from the crude RUS-C were tested, and cytotoxicity was monitored using the NF-κ.B reporter cell. It was found that inhibitory activity appeared in RUS-C20 () and RUS-C40 (). RUS-C20 showed stronger potency than RUS-C40. RUS-C00, RUS-C60, and RUS-C100 showed no inhibitory activity in the NF-κ.B reporter assay (data not shown). RUS-C100 was cytotoxic but the rest of the fractions were not. The first round of fractionation resulted in separation of the inhibitory activity from the cytotoxic activity.
With the guidance of the NF-κ.B reporter assay, the potent fraction, RUS-C20, was further fractioned. A Sephadex LH-20 column was employed, and 16 new fractions were obtained. Based on the HPLC analyses, fractions that were similar in chromatrographic composition were combined. As a result, fractions 1 to 6 were combined to yield 65.0 mg of RUS-C20A, fractions 7 to 10 were combined to yield 5.1 mg of RUS-C20B, and fractions 11 to 16 were combined to yield 5.0 mg of RUS-C20C.
Fraction RUS-C20A contained three major compounds () that eluted at 3.19, 4.01, and 4.72 min. Fraction RUS-C20B contained two major compounds, which eluted around 4.2 and 5.77 min. Fraction RUS-C20C featured a group of four compounds eluting at 2.88, 4.30, 5.17, and 8.00 min. Another compound in this fraction eluted at 16.33 min.
Figure 6 Chromatographic fingerprints of the active fraction RUS-C20 (a) and its three subfractions, RUS-C20A (b), RUS-C20B (c) with insets of UV spectra for two compounds (peak 1 and peak 2), and RUS-C20C (d) with insets of UV spectra of two distinct compound groups (group 1: peaks 3, 4, 5 and 6; group 2: peak 7).

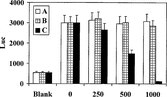
All the three subfractions (RUS-C20A, RUS-C20B, and RUS-C20C) obtained from the active fraction RUS-C20 were tested using the NF-κ.B reporter assay. The results indicated that only the RUS-C20C subfraction retained inhibitory activity ().
Figure 7 NF-κ.B inhibition by subfractions of RUS-C20 (µg/ml). Vertical lines on the top of bars represent one standard deviation of the mean value from triplicates.
The active compound(s) retained in RUS-C20C might be gallotannins judging by their UV absorption spectra compared with pure gallic acid, the basic metabolite of this class.
Discussion
Botanicals are widely used in the treatment and prevention of disease. Efficacy, bioactive component, and mechanism of action are three major aspects in studying medical applications of botanicals. In this study, we examined the bioactivity of the leaf extract of Rubus suavissimus. using a NF-κ.B reporter and conducted bioassay-guided fractionation of the crude extract. Results indicated the leaf extract of Rubus suavissimus. exhibits inhibitory activity of NF-κ.B. More importantly, this activity can be separated from cell toxicity through chromatographic fractionation. This NF-κ.B inhibition activity might contribute to the therapeutic function of the leaf extract of Rubus suavissimus. in some chronic diseases such as hypertension and type 2 diabetes. Our previous studies consistently support that inhibition of NF-κ.B leads to improvement of glucose metabolism (Gao et al., Citation2003Citation2004). The leaf tea of this plant has been used as a folk remedy to relieve diabetic symptoms (Deng, Citation1997), despite the lack of literature depicting the possible mechanisms. Inhibition of NF-κ.B of this leaf extract could be one of the mechanisms that support the folk use. A further investigation of the nontoxic active fractions is warranted in establishing the link between in vitro. assay and an in vivo. response. If such a link is confirmed, then the NF-κ.B assay may become an important tool in directing the standardization process of this leaf extract toward human clinical studies in diabetic patients.
For a reliable therapeutic activity of botanicals, it is common practice to standardize the bioactive compounds. In this study, we demonstrated that the NF-κ.B inhibitory activity could be retained in a refined fraction and this activity could be separated from cytotoxicity. The positive activity of crude material was enriched through the fractionation process. The undesirable components such as antagonists, if they exist, may also be separated through this bioactivity-directed fractionation process. Our data suggest that the NF-κ.B reporter cell line may be used as a quick and accurate functional assay for screening large numbers of botanical samples. Future work should be focused on characterizing the bioactive fraction and using the active compounds to standardize the leaf extract of R. suavissimus.. This standardized extract can then go through in vivo. evaluation without concerns of reliability, which is often seen as a flaw in the nutraceutical industry.
References
- Baldwin AS, Jr (2001): Series introduction: The transcription factor NF-kappaB and human disease. J Clin Invest 107: 3–6. [PUBMED], [INFOTRIEVE], [CSA]
- Deng S-L (1997): Production and utilization of Rubus suavissimus. S. Lee. Guangxi Forestry Science 26: 91–92. [CSA]
- Gao F, Chen F-H, Tanaka T, Kasai R, Seto T, Tanaka O (1985): 19α-Hydroxyursane-type triterpene glucosyl esters from the roots of Rubus suavissimus. S. Lee. Chem Pharm Bull 33: 37–40. [CSA]
- Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon M, Ye J (2002): Serine phosphorylation of insulin receptor substrate 1 (IRS-1) by inhibitor kappaB kinase (IKK) complex. J Biol Chem 277: 48115–48121. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Gao Z, Zhang X, Hwang D, Lefevre M, Ye J (2004): Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol 18: 2024–2034. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Gao Z, Zuberi A, Quon M, Dong Z, Ye J (2003): Aspirin inhibits TNF-induced serine phosphorylation of IRS-1 through targeting multiple serine kinases. J Biol Chem 278: 24944–24950. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hirono S, Chou W-H, Kasai R, Tanaka O, Tada T (1990): Sweet and bitter diterpene-glucosides from leaf of Rubus suavissimus.. Chem Pharm Bull 38: 1743–1744. [CSA]
- Huang P-F, Jiang S-Q (2002): Comprehensive utilization of Rubus suavissimus. S. Lee. Guangxi Huagong 31: 24–25. [CSA]
- Kitahata S, Ishikawa H, Miyata T, Tanaka O (1989): Production of rubusoside derivatives by transgalactosylation. Agric Biol Chem 53: 2923–2928. [CSA]
- Kotaro U (1997): Antiallergy action of Rubus suavissimus.. Shokuhin Kogyo 40: 52–59. [CSA]
- Nakahara K (1998): Anti-allergic activity of Tiencha and oolong tea polyphenols. Food Style 21 2: 45–49. [CSA]
- Nakahara K, Miyagawa K, Kodama T, Fuji W (1996): Anti-allergic composition containing GOD-type ellagitannin as active ingredient. Europe Patent No. 727218.
- Ohtani K, Aikawa Y, Kasai R, Chou W-H, Yamasaki K, Tanaka O (1992): Minor diterpene glycosides from sweet leaf of Rubus suavissimus.. Phytochemistry 31: 1553–1559. [CSA], [CROSSREF]
- Ono Y (2002): The health beneficial effects of Tien-cha (Rubus suavissimus. tea) and its applications. Food Style 21: 9 77–80. [CSA]
- Seto T, Tanaka T, Naruhashi N (1984): β-Glucosyl esters of 19α-hydroxyursolic acid derivatives in leaf of Rubus. species. Phytochemistry 23: 2829–2834. [CSA], [CROSSREF]
- Tanaka T, Kohda H, Tanaka O, Chen F-H, Chou W-H, Leu J-L (1981): Rubusoside (β-D-glucosyl ester of 13-O.-β-D-glucosyl-steviol), a sweet principle of Rubus chingii. Hu (Rosacease). Agric Biol Chem 45: 2165–2166. [CSA]
- Zhang X, Ye J, Wang L, Manosroi J, Shi X, Rojanasakul Y (2001): Rapid and sensitive assay of tumor necrosis factor alpha gene transcription. Pharm Res 18: 408–411. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Zhou W-H, Hirono S, Kasai R, Tanaka O (1992): A new sweet diterpene-glucoside in leaf of Rubus suavissimus.. Acta Botanica Sinica 34: 315–318. [CSA]