Abstract
We were able to establish a suspension culture of Linum boissieri. that produces 6-methoxypodophyllotoxin (6MPT). As a first step to gain insight into the lignan biosynthesis in L. boissieri. cell cultures, we were able to measure phenylalanine ammonia-lyase (PAL) activity in raw protein extracts. PAL is a key enzyme in the early part of the general phenylpropanoid pathway, leading (beside others) to the precursors for lignan biosynthesis.
Introduction
Lignans are phenolic compounds that are widespread in the plant kingdom and show a wide variety of biological activities including antitumor, anti-HIV, immunosuppressive, hipolipidemic, antifungal, phytoestrogenic, and antiasthmatic activities (Charlton, Citation1998; Ward, Citation1999; Rios et al., Citation2002; Apers et al., Citation2003). Lignans are derived from two phenlypropanoid units that are linked by a C–C bond between the side-chain carbon atoms 8 and 8′.
6-Methoxypodophyllotoxin (6MPTOX) and podophyllotoxin (PTOX) belong to the class of aryltetralin lignans. PTOX is the starting compound for the production of three important clinically applied anticancer drugs: etoposide, teniposide, and Etopophos. Until now, PTOX is isolated from roots and rhizomes of wild growing plants of Podophyllum hexandrum., which is endemic in the Himalayan region and now an endangered species. In order to find alternative sources, Linum. species were recognized to contain lignans of various types. Plant in vitro. cultivation has several advantages over collecting plants from the wild or cultivating them on fields (Alfermann et al., Citation2003). Metabolites like lignans can be produced under controlled and reproducible conditions, independent of geographic and climatic factors. It is not necessary to use herbicides or insecticides. Especially, cell suspension cultures can show high growth rates combined with high accumulation of the desired metabolite in a short time. In addition, such cultures can be an excellent source to study the biosynthesis of secondary compounds (Fuss, Citation2003). Feeding of labeled and not-labeled precursors can easily be done, and the precursors are often more efficiently incorporated in in vitro. cultivated plant cells than in whole plants. Because plant in vitro. cultures are available over the whole year and protein extraction from the cultures is usually easier than from soil-grown plants, in vitro. cultivated plant cells are a good source to investigate enzymatic activities (Seidel et al., Citation2002; Fuss, Citation2003). Previous work has shown that suspension and callus of the Linum. s.pecies (Linaceae) are useful for the production and accumulation of podophyllotoxin and 6-methoxypodophyllotoxin (Empt, Citation2000; Petersen, Citation2001). As a part of our ongoing studies on the suspension cultures of Linum boissieri., we identified 6-methoxypodophyllotoxin from this species, which is endemic in Turkey and belongs to section Syllinum.. Generally, aryltetralin-types of lignans have been reported in the section Syllinum.. As first experiments concerning the lignan biosynthesis in this species, we measured the activity of the phenylalanine ammonia-lyase (PAL) that is the entrance enzyme of the general phenylpropanoid pathway leading (beside others) to the precursors of lignan biosynthesis.
Materials and Methods
Plant sample
The plant material from Linum boisseri. was collected in July 2002 near Fethiye in Turkey. A plant specimen was deposited in the herbarium of the Faculty of Pharmacy, University of Ankara. Seeds of L. boissieri. were germinated under sterile conditions on MS medium (Murashige & Skoog) without hormones at 25°C in continuous light (150 µE m−2 s−1). Shoot explants were placed on MS medium solidified with 1% agar-agar. Developing callus tissue was transferred to liquid MS medium containing 0.4 mg naphthalene acetic acid (NAA) in order to establish a suspension culture. Suspension cultures were subcultivated weekly by transferring 5 g cells to 50 ml fresh medium in 250-ml Erlenmeyer flasks. The cultures were placed on a gyratory shaker (120 rpm) in the dark at 25°C. Samples were taken in duplicate at days 7 and 14 of the cultivation period. For determination of fresh weight, cells were separated from the medium by filtration under suction. Dry weight was determined after lyophilization for 4 days.
Extraction and identification of lignan aglycons
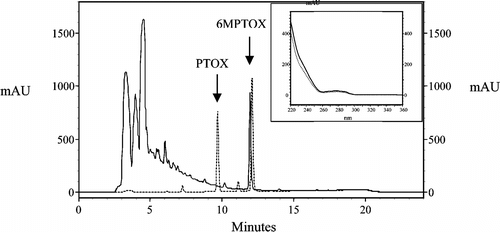
A fine powder (0.2 g) of the lyophilized plant material was extracted with methanol (2 ml) in an ultrasonic bath (two times for 30 s). Distilled water (6 ml) was added, and the pH was adjusted to 5.0 by addition of o.-phosphoric acid. After addition of β.-glucosidase (1 mg) and incubation at 35°C for 1 h, methanol (12 ml) was added and the mixture incubated for another 10 min at 70°C in an ultrasonic bath. After centrifugation, the supernatant was used directly for HPLC, or stored at –18°C. Analysis was performed with a Thermo Quest HPLC system (Egelsbach, Germany) equipped with a photodiode array detector. Separation was performed using a GROM-SIL 120 ODS-5ST column with guard column (250-mm long and 4.6-mm i.d., and 40-mm long and 4.6-mm i.d., respectively; 5-µm particle size; Grom Company, Herrenberg, Germany) and a gradient system with water (A) and acetonitrile (B) as eluents as follows: 0 to 17 min from 40% to 67% B, from 17 to 18 min to 40% B, and until 24 min hold at 40% B. The flow rate increased from 0.8 ml/min at 0 min to 1.0 ml/min at 17.0 min and decreased again to 0.8 ml/min between 18 and 24 min. 6MPTOX was identified by comparison with an authentic standard ().
Figure 1. HPLC analysis (detection at 230 nm) of an extract from Linum boissieri. suspension cells harvested on day 14 showing 6-methoxypodophyllotoxin (6MPTOX) at Rt 11.8 min. (solid line) in comparison with an authentic standard of podophyllotoxin (Rt 9.8 min) and 6MPTOX (dotted line). The insert shows the comparison of spectra of the 6MPTOX from the plant extract (solid line) and the standard (dotted line).
Phenylalanine ammonia-lyase studies
Cells (5 g) were homogenized three times with 1 g Polyclar 10 and 5 ml of extraction buffer (0.1 M Tris/HCl, 1 mM DTT, pH 7.5) with the help of a hand-held dispersion tool (Ultra Turrax T25, Ika, Staufen, Germany) with 20,000 rpm for 30 s each with breaks of 30 s on ice. The homogenate was centrifuged (48,000 × g, 20 min) and the supernatant filtered through glass wool. The filtrate was used as the crude enzyme extract. Protein concentration was determined in the filtrate according to Bradford (Citation1976). Bovine serum albumine (1 mg/ml) served as a standard. Phenylalanine ammonia-lyase activity was assayed by monitoring the formation of cinnamic acid as described by Zimmermann and Hahlbrock (Citation1975).
Results and Discussion
We were able to establish a well-growing cell suspension culture of L. boissieri. reaching 1.19±0.06 g/50 ml dry weight within 7 days and 1.03±0.19 g/50 ml dry weight within 14 days of cultivation, which is slightly higher than the dry weight reached with a cell suspension culture of L. album. (about 0.90 mg/50 ml at day 7 and 0.65 mg/50 ml at day 14). The L. boissieri. cells accumulated 2.61±0.04 mg 6MPTOX/g dry weight until day 7 and 3.40±0.02 mg/g dry weight until day 14. This is three times more than we could isolate from roots of L. boissieri. from which we isolated 0.09% of the dry weight 6MPTOX as its glucoside (Konuklugil, Citation1996).
As a first experiment concerning the 6MPTOX biosynthesis in the cell cultures of L. boissieri., we measured PAL activity in raw extracts of L. boissieri. cells from the fourth day of cultivation when PAL activity reached 0.790 mkat/kg protein, which is in the same range of activity we measured from a suspension culture of L. album., which accumulates up to 8 mg/g dry weight 6MPTOX within 10 days of cultivation. These preliminary data show that suspension cultures of L. boissieri. are suitable for enzymological studies concerning lignan biosynthesis.
Acknowledgments
Financial support by EC-project BIO-4-CT 98–0451 “Lignocancer” and by BMB F [Bonn and Tübitak (Ankara)] for cooperation within the project 42.6.KoA.6.B (Cytotoxic Lignans from Turkish Plants), University of Ankara, Biotechnology Institute (2002-K-120–130-2), is gratefully acknowledged.
References
- Alfermann AW, Petersen M, Fuss E (2003): Production of natural products by plant cell biotechnology: Results, problems and perspectives. In: Laimer M, Rücker W, eds., Plant Tissue Culture, 100 years since Gottlieb Haberlandt. Wien, New York, Springer.
- Apers S, Vlietinck, Pieters L (2003): Lignans and neolignans as lead compounds. Phytochemistry Rev 2: 307–321. [CSA]
- Bradford MM (1976): A rapid and sensitive method for the ouantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72: 248–254. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Charlton JM (1998): Antiviral cctivity of lignans. J Nat Prod 61: 1447–1451. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Empt U, Alfermann WA, Pras N, Petersen M (2000): The use of plant cell cultures for the production of podophyllotoxin and related lignans. J App Bot 74: 145–150. [CSA]
- Fuss E (2003): Lignans in plant cell and organ cultures: An overview. Phytochem Rev 2: 307–321. [CSA], [CROSSREF]
- Konuklugil B (1996): Aryltetralin lignans from genus Linum.. Fitoterapia 67: 379–381. [CSA]
- Petersen M, Alfermann AW (2001): The production of cytotoxic lignans by plant cell cultures. Appl Microbiol Biotechnol 55: 135–142. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Rios JL, Giner RM, Prieto JM (2002): Phytoestrogen and lignans: Bioactivity of lignans. Studies Nat Prod 26: 183–292. [CSA]
- Seidel V, Windhövel J, Eaton G, Alfermann AW, Arroo RRJ, Medarde M, Petersen M, Wooley JG (2002): Biosynthesis of podophyllotoxin in Linum album. cell cultures. Planta 215: 1031–1039. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Thompson LU, Seidl MM, Rickard SE, Orcheson LJ, Fong HHS (1996): Antitumourigenic effect of a mammalian lignan precursor from flaxseed. Nutr Cancer 26: 159–165. [PUBMED], [INFOTRIEVE], [CSA]
- Ward RS (1999): Lignans, neolignans and related compounds. Nat Prod Rep 16: 75–96. [CSA], [CROSSREF]
- Zimmermann A, Hahlbrock K (1975): Light induced changes of enzyme activities in parsley cell-suspension cultures—Purification and some properties of phenylalanine ammonia-lyase (EC 4315). Arch Biochem Biophys 166: 54–62. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]