ABSTRACT
The content of herniarin, (Z.)- and (E.)-2-β.-D-glucopyranosyloxy-4-methoxycinnamic acid, umbelliferone, chlorogenic acid, and (Z.)- and (E.)-ene-yne-dicycloether was evaluated in Matricaria chamomilla.L. leaf rosette in relation to the leaf age. Developmental changes of the herniarin quantity in diploid and tetraploid cultivar were similar. The highest content of (E.)-2-β.-D-glucopyranosyloxy-4-methoxycinnamic acid was found in the youngest leaves. The amount of umbelliferone and chlorogenic acid increased with the leaf age. Differences in amount of (Z.)- and (E.)-ene-yne-dicycloether between tetraploid and diploid plants were significant in all leaf pairs. However, their content during the leaf development was not considerably changed, contrary to coumarins.
Introduction
The initiation of leaf differentiation from the shoot apical meristem during plant development and its arrangement and shape are controlled by a genetic pattern termed phyllotaxy. (Hake & Jackson, Citation1995). The content of secondary metabolites is also modified by resizing and functional maturity in developing leaves. It often depends on enzymatic processes in plant tissues after the corresponding gene expression (Gross et al., Citation2002).
Matricaria chamomilla. L. (Asteraceae) is a widely known medicinal plant used mainly for its anti-inflammatory and spasmolytic activities, which are attributed to the various secondary metabolites (Schilcher, Citation1987). Apart from flavonoids, sesquiterpenes, and polyacetylenes, coumarins are also important constituents of chamomile. Polyploidization is one of the methods for increasing production potential. A higher content of active substances was expected in tetraploid types of healing plants in comparison to diploid ones. Matricaria chamomilla. tetraploid cultivars are characterized by a higher yield of flower drug than diploid plants, but studies of increases of secondary product content have been ambiguous (Chládek & Rod, Citation1958; Svehlíková et al., Citation2000). Differences between chamomile cultivars were also confirmed by Seider-Lozykowska (Citation2003) in some morphological trains (e.g., flowerhead diameter, 100-flowerhead weight, stoma length).
The objective of this study was to evaluate the dynamics of production of the coumarins and some other metabolites and examine differences in the patterns of quantity during development of leaf rosette in chamomile cultivars with different ploidy levels.
Materials and Methods
Plants of tetraploid cultivar “Lutea” and diploid cultivar “Novbona” of Matricaria chamomilla. L. were cultivated individually in plastic pots filled with soil under laboratory conditions (12-h photoperiod, soil moisture 60% of water-holding capacity). Nine-week-old plants were collected in the stage of leaf rosette. Individual leaves (12) from the oldest (outer) to the youngest (inner) ones were taken from the rosette of each plant and dried (110°C, 1.5 h). Samples consisted of a pair of subsequent leaves from each plant. Methanol extracts of dry homogenized leaves were prepared. Gradient high performance liquid chromatography (HPLC) was used to estimate studied compounds [column Tessek SGX C18 7 µm (4 × 250, Prague, Czech Republic), flow rate 0.8 mL/min]. The mobile phase was a mixture of A, acetonitrile:water:CF3COOH (19:80:0.007); B, acetonitrile:water (4:6); and C, acetonitrile:water (9:1). All solvents were gradient grade (Merck, Darmstadt, Germany). The elution program was from 100% A to 100% B (25 min), from 100% B to 100% C (5 min), isocratic 100% C (5 min), and from 100% C to 100% A (10 min). Detection was performed at 320 nm. Chlorogenic acid (Fluka, Buchs, Switzerland), herniarin (Extrasynthese, Genay, France), and umbelliferone (Merck) standard compounds were used for the quantification. Herniarin glucoside precursors (Z.)- and (E.)-2-β.-D-glucopyranosyloxy-4-methoxycinnamic acids (GMCA) and (Z.)- and (E.)-ene-yne-dicycloethers were prepared and identified as described in previous studies (Repcák et al., Citation1999Citation2001). (Z.)- and (E.)-ene-yne-Dicycloethers were evaluated as a sum of both isomers.
All statistical analyses were performed using ANOVA (LSD, multiple range test). Experiments were conducted with 20 plants of each cultivar.
Results and Discussion
Chamomile rosette leaves of diploid and tetraploid plants significantly differed in dry weight. As may be seen from , the first to fourth mature leaves were lower in weight and size than the fifth to eighth ones. The next four leaves were immature in development.
Table 1 The changes of leaf pairs dry weight (mg) of tetraploid and diploid Matricaria chamomilla. plants from the oldest outer leaves (1 + 2) to the youngest inner leaves (11 + 12).
The content of estimated metabolites of Matricaria chamomilla. leaf rosette was changed in relation to the leaf age (Figs. and ). The course of quantitative changes of herniarin in diploid and tetraploid cultivar was similar (Fig.). Its maximum content was observed in the leaf pair 9 + 10: 0.26 mg/g d.m. in tetraploid cultivar and 0.15 mg/g dry mass (d.m.) in diploid one.
Figure 1 Changes of (a) herniarin, (b) umbelliferone, (c) chlorogenic acid, and (d) ene-yne-dicycloether in the leaf pairs of tetraploid (T) and diploid (D) Matricaria chamomilla. plants during leaf rosette ontogeny from the oldest outer leaves (1 + 2) to the youngest inner leaves (11 + 12). Points sharing the same letters are not significantly different (LSD test, p = 0.05).
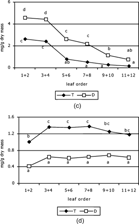
Figure 2 Changes of (Z.)- and (E.)-2-β.-D-glucopyranosyloxy-4-methoxycinnamic acids in the leaf pairs of tetraploid and diploid Matricaria chamomilla. plants during leaf rosette ontogeny from the oldest outer leaves (1 + 2) to the youngest inner leaves (11 + 12). Points sharing the same letters are not significantly different (LSD test, p = 0.05).

In regard to the content of (Z.)- and (E.)-2-β.-D-glucopyranosyloxy-4-methoxycinnamic acid, the glucoside precursor of herniarin, no significant differences between cultivars were observed (Fig.). It is remarkable that the (E.)-isomer content increased and its maximum amount in the youngest leaves was approximately 6-times higher than that of the (Z.)-isomer.
The amount of umbelliferone increased with the leaf age in tetraploid as well as in diploid chamomile with maximum in the oldest leaves (Fig.). Higher content of umbelliferone in 1 + 2 and 3 + 4 leaf pairs of diploid plants in comparison with tetraploid ones was statistically significant.
Similar patterns of accumulation of (Z.)- and (E.)-2-β.-D-glucopyranosyloxy-4-methoxycinnamic acid and its product herniarin in the youngest leaves support the thesis about their importance in defense of the immature organ. A distinct role in defense may be proposed for umbelliferone, which was identified as phytoalexin in some species of plants (Afek et al., Citation1999; Clerivet & Alami, Citation1999).
The dynamics of chlorogenic acid content is displayed in . Its maximum was observed in the oldest leaves and the minimum in the youngest leaves. Higher content of chlorogenic acid was found in diploid plants (from 4.6 mg/g d.m. to 0.77 mg/g d.m.) compared to tetraploid cultivar (from 2.7 mg/g d.m. to 0.2 mg/g d.m.). These differences were statistically significant in the range from the oldest leaves 1 + 2 to the leaf pair 9 + 10. Chlorogenic acid and its isomers levels were increased in apoplast of tobacco leaves during ageing (Takahama et al., Citation1999).
All leaves of tetraploid cultivar contained more (Z.)- and (E.)-ene-yne-dicycloether (from 0.99 mg/g d.m. to 1.37 mg/g d.m.) than those of diploid one (from 0.41 mg/g d.m. to 0.68 mg/g d.m.; Fig.). Differences in amount of (Z.)- and (E.)-ene-yne-dicycloether between tetraploid and diploid plants in all leaf pairs were statistically significant. On the other hand, the content of dicycloether was not considerably changed during leaf development, contrary to coumarins. This polyacetylene is a specific constitutive secondary metabolite of chamomile, present in all organs (Reichling et al., Citation1984).
Published data about quantitative changes of secondary metabolites during ageing are infrequent. Developmental changes of the monoterpene composition were studied in parts of Mentha. × piperita. leaves of different age. The analyses showed that all these dynamic changes started at the distal extremity of the leaf and shifted progressively toward the base (Voirin & Bayet, Citation1996). The changes in glucosinolates sinigrin and glucoraphanin contents were found in Brassica. species during plant development. Sinigrin concentration in B. juncea. (L.) Czern. and B. nigra. (L.) Koch. decreased from seedling to early flowering stage, increased in the late flowering stage, and then decreased again during the seed maturation. The concentration of glucoraphanin in B. oleracea. var. italica. Plenck decreased from the start of seed germination to the flowering stages (Rangkadilok et al., Citation2002).
The changes of secondary metabolite patterns during leaf rosette development of Matricaria chamomilla. have not been studied. However, phases of anthodia development were often chosen for evaluation of some metabolite accumulation. Repcák et al. (Citation1998) determined the content of herniarin after acid hydrolysis of (Z.)- and (E.)-2-β.-D-glucopyranosyloxy-4-methoxycinnamic acid in four phases of anthodia. The percentage of the compound decreased significantly during the ontogenesis of anthodia both in diploid and tetraploid varieties of chamomile.
Differences in secondary metabolites (herniarin, umbelliferone, chlorogenic acid) content between diploid and tetraploid cultivars found in some developmental stages of leaves were similar to differences of essential oil content in developing anthodia (Repcák et al., Citation1993). On the other hand, independence of (Z.)- and (E.)-2-β.-D-glucopyranosyloxy-4-methoxycinnamic acid content on ploidy in leaves was in agreement with our previous results concerning anthodia (Repcák et al., Citation2001).
Acknowledgments
This work was supported by the grant agency VEGA, Slovakia (grant no. 1/0444/03). The authors wish to thank Mrs. Anna Michalcová for her valuable technical assistance.
References
- Afek U, Orenstein J, Carmeli S, Rodov V, Joseph MB (1999): Umbelliferone, a phytoalexin associated with resistance of immature Marsh grapefruit to Penicillium digitatum.. Phytochemistry 50: 1129–1132.
- Chládek M, Rod J (1958): Importance of polyploidy in breeding of Matricaria chamomilla. L. (in Czech). Rostlinná Výroba 4: 305–316.
- Clerivet A, Alami I (1999): Effects of jasmonic acid and of an elicitor from Ceratocystis fimbriata. F. sp platani. on the accumulation of phytoalexins in leaves of susceptible and resistant plane trees. Plant Sci 148: 105–110. [CROSSREF]
- Gross M, Friedman J, Dudai N, Larkov O, Cohen Y, Bar E, Ravid U, Putievsky E, Lewinsohn E (2002): Biosynthesis of estragole and t.-anethole in bitter fennel (Foeniculum vulgare. Mill. var. vulgare.) chemotypes. Changes in SAM: Phenylpropene O.-methyltransferase activities during development. Plant Sci 163: 1047–1053. [CROSSREF]
- Hake S, Jackson D (1995): Genetic and molecular analysis of patterning in plant development. ASGSB Bull 8: 29–37. [PUBMED], [INFOTRIEVE], [CSA]
- Rangkadilok N, Nicolas ME, Bennett RN, Premier RR, Eagling DR, Taylor WJ (2002): Developmental changes of sinigrin and glucoraphanin in three Brassic.a species (Brasicca nigra., Brasicca juncea. and Brasicca oleracea. var. italica.). Sci Hort 96: 11–26. [CROSSREF]
- Reichling J, Bisson W, Becker H (1984): Vergleichende untersuchungen zur bildung und akkumulation von etherischen öls in der intakten pflanze und in der calluskultur von Matricaria chamomilla.. Planta Med 50: 285–364.
- Repcák M, Cernaj P, Mártonfi P (1993): The essential oil content and composition in diploid and tetraploid Chamomilla recutita. during the ontogenesis of anthodia. J Essent Oil Res 5: 297–300. [CSA]
- Repcák M, Eliasová A, Ruscancinová A (1998): Production of herniarin in diploid and tetraploid Chamomilla recutita.. Pharmazie 53: 278–279.
- Repcák M, Imrich J, Garcár J (1999): Quantitative evaluation of the main sequiterpenes and polyacetylenes of Chamomilla recutita. essential oil by high-performance liquid chromatography. Phytochem Anal 10: 335–338. [CROSSREF]
- Repcák M, Pastírová A, Imrich J, Svehlíková V, Mártonfi P (2001): The variability of (Z.)- and (E.)-2-β.-D-glucopyranosyloxy-4-methoxy cinnamic acids and apigenin glucosides in diploid and tetraploid Chamomilla recutita.. Plant Breeding 120: 188–190. [CROSSREF]
- Schilcher H (1987): Die Kamille. Stuttgart, Wiss. Verlag, pp. 152.
- Seider-Lozikowska K (2003): Determination of the ploidy level in chamomile (Chamomilla recutita. (L.) Rausch.) strains rich in α.-bisabolol. J Appl Genet 44: 151–155.
- Svehlíková V, Repcák M (2000): Variation of apigenin quantity in diploid and tetraploid Chamomilla recutita. (L.) Rauschert. Plant Biol 2: 403–407. [CROSSREF]
- Takahama U, Hirotsu M, Oniki T (1999): Age-dependent changes in levels of ascorbic acid and chlorogenic acid, and activities of peroxidase and superoxide dismutase in the apoplast of tobacco leaves: Mechanism of the oxidation of chlorogenic acid in the apoplast. Plant Cell Physiol 40: 716–724.
- Voirin B, Bayet C (1996): Developmental changes in the monoterpene composition of Mentha. × piperita. leaves from individual peltate trichomes. Phytochemistry 43: 573–580. [CROSSREF]
