ABSTRACT
Previous studies have shown that the ethanol extract of Plumbago zeylanica. L. stems (EPZ) suppresses immediate allergic reactions. We extended these studies to examine the effect of EPZ on delayed-type hypersensitivity reactions. Our results showed that EPZ (500 and 1000 mg/kg), orally administered during either sensitization stage or effector stage, effectively attenuated picryl chloride–induced delayed-type hypersensitivity (PC-DTH) in mice. In vitro., EPZ (100 and 200 µg/ml) concentration-dependently inhibited Con A–induced splenocyte proliferation and interleukin-2 (IL-2) production but had little effect on nitric oxide (NO) production from murine peritoneal macrophages activated with lipopolysaccharide (LPS). EPZ (500 and 1000 mg/kg) also showed a significant inhibition of ear swelling caused by xylene in mice. These results suggested that EPE alleviated delayed-type hypersensitivity in mice, which might be attributable to inhibition of proliferation and differentiation of T lymphocytes as well as anti-inflammatory activities.
Introduction
Plumbago zeylanica. L. (Plumbaginaceae) is a tropical shrub. In China, it mainly grows in the southern provinces, including Guangdong, Guangxi, Fujian, Sichuan, and Yunnan. In traditional Chinese medicine, the roots and leaves of P. zeylanica. L. are usually used to treat rheumatism, intestinal parasites, anemia due to stagnant blood, external and internal trauma, toxic swelling, and malignant furunculous scabies (Jiangsu New Medical College, Citation1979). Previous studies have shown that P. zeylanica. extract possesses antiplasmodial (Simonsen et al., Citation2001), antimicrobial (Ahmad et al., Citation2000), antifungal (Mehmood et al., Citation1999), anti-inflammatory (Oyedapo, Citation1996), antihyperglycemic (Olagunju, Citation1999), hypolipidemic and antiatherosclerotic activities (Sharma et al., Citation1991). The 70% ethanol extract from P. zeylanica. stems (EPZ) effectively inhibited mast cell–dependent immediate allergic reactions (Dai et al., Citation2004). The objective of the current work was to investigate the effect of EPZ on cellular immunity–mediated delayed-type hypersensitivity reactions caused by picryl chloride in mice and assess the possible mechanism of action.
Materials and Methods
Animals
Male ICR mice weighing 24 ∼ 26 g were obtained from the experimental animal center of China Pharmaceutical University (Nanjing, China), and BALB/c mice weighing 20 ∼ 22 g were obtained from Sino-British Sippr/Bk Lab Animal Co. Ltd. (Shanghai, China). All animals were fed standard Laboratory chow and water ad libitum.. The environment was maintained at 22±2°C with a 12 h light and dark cycle. Animal experiments were conducted in accordance with the current ethical regulations on animal research of China Pharmaceutical University, and all animals used in experiments received humane care.
Chemicals and reagents
Concanavalin A (Con A), 3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide (MTT), naphthlyenediamine dihydrochloride, sulfanilamide, and lipopolysaccharide (LPS) were purchased from Sigma (USA). Picryl chloride (PC) was purchased from Tokyo Kassei Industry Co. Ltd (Japan). Methyl-α-D-mannopyranoside (α-MM) was purchased from Fluka. Dexamethasone acetate was purchased from Zhejiang Xianju Pharmaceutical Co. Ltd (Xianju City, Zhejiang, China).
Preparation of plant extract
Samples of P. zeylanica. were purchased from the herbal market in Hong Kong, China, in October 2001. Its identity was confirmed as the stem of P. zeylanica. L. by anatomical and thin-layer chromatography (TLC) analysis as well as comparison with authentic specimens kept at the Herbarium, Department of Biology, the Chinese University of Hong Kong. A voucher specimen (But 0106) is deposited in the Museum of Institute of Chinese Medicine, The Chinese University of Hong Kong. P. zeylanica. stems (200 g) were ground and refluxed with 70% ethanol (1.5 L) three-times for 1 h. After filtration with filter paper, the clear supernatants were concentrated under reduced pressure at 45°C in a vacuum rotary evaporator and lyophilized to give a dry extract (11.8 g). The extract (EPZ) was freshly suspended in distilled water or medium before use.
PC-induced delayed-type hypersensitivity (PC-DTH)
ICR mice were sensitized by applying 100 µl of 1% PC dissolved in ethanol to the abdominal skin, shaved 24 h before. After 5 days, animals were challenged by painting 30 µl of 1% PC dissolved in olive oil on the right ear lobes. The thickness of ear lobes was measured 24 h after the challenge using an engineer's micrometer, and ear swelling was represented as the thickness difference between right lobes and left ones. Test samples and dexamethasone were orally administered for 6 consecutive days from sensitization to challenge (sensitization stage) or for two-times immediately and at 16 h after challenge (effector stage), respectively.
Preparation of splenocytes
Spleens were taken from normal BALB/c mice and dissociated into a single cell suspension in 5 ml RPMI-1640 (Gibco, USA) supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM L-glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 µg/ml streptomycin (Sigma). The suspension was centrifuged at 200 g for 5 min, and the supernatant was discarded. Erythrocytes were destroyed with 0.17 M Tris (hydroxymethylaminomethane) 0.75% NH4Cl solution. After washing with RPMI-1640 twice, cells were counted by Trypan blue exclusion and adjusted to needed density.
Cytotoxicity on splenocytes
Splenocyte suspension (5 × 106 cells/ml) (100 µl) was incubated in 96-well plates at 37°C in 5% CO2 humidified air for 68 h with the same volume of test samples dissolved in RPMI-1640 with concentrations varying from 50 to 400 µg/ml. Then, 20 µl of MTT (5 mg/ml) were added for an additional 4 h incubation. After termination of incubation, the plates were centrifuged at 1000 g for 20 min. Aspirating the supernatants, 200 µl of dimethyl sulfoxide was added into each well to dissolve the formazan particles, and the absorbance at 540 nm (OD540) was measured using a Model 450 Micro-plate Reader (Bio-Rad, Hercules CA, USA).
Con A–induced splenocyte proliferation
The proliferation of splenocytes responding to Con A stimulation was evaluated using the MTT assay (Lombardi et al., Citation1991). In brief, 100 µl of splenocyte suspension (5 × 106 cells/ml) was incubated with 50 µl of Con A (final concentration 5 µg/ml) and 50 µl of various concentrations of samples in 96-well plates at 37°C in 5% CO2 humidified air for 68 h, and then 20 µl of MTT (5 mg/ml) was added for an additional 4 h incubation. As described in the cytotoxic test, OD540 values of each well was determined. The proliferation index was expressed as the ratio of OD540 value of Con A–stimulated cells and that of nonstimulated cells.
Con A–induced Il-2 production
In 24-well plates, 1 ml of splenocyte suspension (5 × 106 cells/ml) was cultured in medium alone or in medium containing Con A (final concentration 5 µg/ml) in the presence or absence of samples for 48 h at 37°C in 5% CO2 incubator. After the termination of incubation, the plates were centrifuged at 1000 g for 20 min, and the supernatants were harvested for IL-2 activity assay. The biological activity of IL-2 was assessed by the activated-splenocyte proliferation assay (Du & Li, Citation1998) with a slight modification. Briefly, splenocytes (2 × 106 cells/ml) from normal BALB/c mice were incubated with a suboptimal dose of Con A (final concentration 3 µg/ml) for 72 h to get modified activated splenocytes. The latter (1 × 106 cells/ml) (100 µl) was incubated with the same volume of samples containing IL-2 and 20 µl of methyl-α-D-mannopyranoside (α-MM) dissolved in sterile PBS in 96-well plates at 37°C in 5% CO2 humidified air for 20 h, and then 20 µl of MTT (5 mg/ml) was added for an additional 4 h incubation. IL-2 activity was represented as the proliferation index on normal splenocytes.
LPS-induced nitric oxide production of murine peritoneal macrophages
Murine resident peritoneal exudate cells were harvested from BALB/c mice by lavaging with ice-cold RPMI-1640 supplemented with 1% heat-inactivated fetal calf serum 4 days after intraperitoneal injection of 2 ml of 0.3% thyoglycollate broth (Wako Junyaku, Japan) (Terawaki et al., Citation1997). Cells were centrifuged at 4°C for 7 min at 150 × g and resuspended in RPMI-1640 supplemented with 10% fetal calf serum. After counting by Trypan blue exclusion, cells were then seeded in 96-well cluster plates at a density of 2 × 106 cells/ml. After 2 h incubation at 37°C in 5% CO2 humidified air, nonadherent cells were removed by washing twice with medium. The remained cells were incubated with medium containing various concentrations of samples and LPS (10 µg/ml) at 37°C for 24 h. The supernatants were harvested for immediate determination of nitric oxide (NO).
NO produced by activated macrophages reacts rapidly with oxygen to produce nitrite. Therefore, nitrite levels in the supernatants of macrophage cultures were measured using Griess reaction (Zinyama et al., Citation2001). The reactions were performed in triplicate by addition of 100 µl of fresh supernatant to 100 µl of Griess reagent (0.1% naphthlyenediamine dihydrochloride/1% sulfanil-amide/2.5% H3PO4). After incubation at room temperature for 15 min, the absorbance was read at 570 nm using a Model 450 Micro-plate Reader (Bio-Rad).
Xylene-induced ear swelling in mice
Acute ear swelling was elicited by applying 30 µl of xylene to the right ears of ICR mice. Thickness of ear lobes was measured 2 h after the elicitation by using an engineer's micrometer. Ear swelling was represented as the thickness difference between right and left lobes. EPZ and indomethacin were orally administered 1 h before the elicitation of xylene.
Statistical analysis
The data are presented as means ±SD. Statistical significance between groups was calculated by analysis of variance of the Student's t.-test. p values less than 0.05 (p < 0.05) were considered significant.
Results
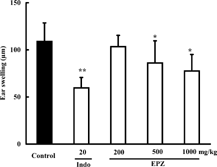
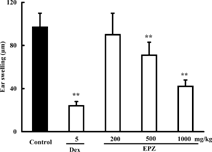
EPZ 200, 500, 1000 mg/kg, orally administered from the day of immunization to the day of challenge (sensitization stage), attenuated PC-DTH in mice by 10.6%, 45.5%, and 65.8%, respectively. Significant differences were observed at doses of 500 and 1000 mg/kg. Dexamethasone, a positive control drug, inhibited PC-DTH by 74.6% at 5 mg/kg (Fig.). EPZ (500, 1000 mg/kg) and dexamethasone (5 mg/kg), administered immediately and at 16 h after PC challenge (effector stage), also effectively alleviated PC-DTH by 26.8%, 56.7%, and 75.3%, respectively (Fig.).
Figure 1. Effects of EPZ and dexamethasone (Dex) administered during sensitization stage on PC-DTH in mice. Data are means ± SD of 9 mice. **p. < 0.01.
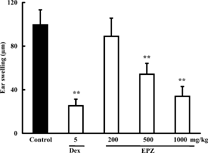
Figure 2. Effects of EPZ and dexamethasone (Dex) administered during effector stage on PC-DTH in mice. Data are means ± SD of 9 mice. **p. < 0.01.
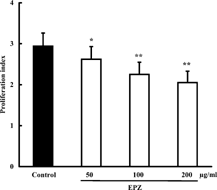
By the MTT assay, we demonstrated that EPZ was absent of cytotoxicity on murine splenocytes at concentrations of 50, 100, 200, and 400 µg/ml (data not shown). EPZ (100, 200 µg/ml), cultured with murine splenocytes for 72 h, showed a concentration-dependent alleviation of Con A–induced splenocyte proliferation. The inhibition percentages were 12.2% and 30.9%, respectively (). Concurrently, EPZ (50, 100, 200 µg/ml) remarkably reduced Con A–elicited IL-2 production from murine splenocytes with inhibition percentages of 10.9%, 23.5% and 30.3% respectively ().
Figure 3. Effect of EPZ on Con A–induced murine splenocyte proliferation. Data are means ± SD of three experiments. *p. < 0.05, **p. < 0.01.
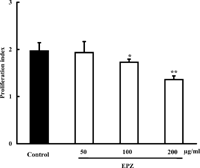
Figure 4. Effect of EPZ on Con A–induced IL-2 production from murine splenocytes. Data are means ± SD of three experiments. *p. < 0.05, **p. < 0.01.
EPZ (100, 200, 400 µg/ml) showed no significant influence on nitric oxide production from murine peritoneal macrophages stimulated with LPS (data not shown).EPZ (500, 1000 mg/kg), orally administered 1 h before the elicitation of xylene in mice, inhibited ear swelling by 21 and 28.8% ().
Discussion
Delayed-type hypersensitivity induced by picryl chloride (PC-DTH) in mice is a typical in vivo. manifestation of T cell–mediated immunity. It is characterized by activation and recruitment of T lymphocytes and macrophages and by resultant ear swelling at 24 to 72 h. DTH reaction can be routinely divided into two main stages; namely, the sensitization stage and the effector stage. In the former stage, primary exposure of antigen-specific T cells to peptide-MHC present on antigen-presenting cells (e.g., Langerhans cells in skin) results in proliferation of T cells and differentiation to type 1 helper T lymphocytes (Th1), which are driven by cytokines such as IL-2. In the latter stage, re-exposure of Th1 cells to antigen elicits the activation of Th1 cells and production of cytokines, notably chemokines and IFN-γ., which recruit and activate mononuclear cells (mostly macrophages). Lysosomal enzymes, peroxide, and superoxide radicals such as nitric oxide produced by infiltrated macrophages, contribute predominantly to the eventual outcome of DTH reaction (exudation of plasma and local tissue damage) (MacMicking et al., Citation1997).
In the current study, we demonstrated that the ethanol extract from Plumbago zeylanica. stems (EPZ), administered during either the sensitization or effector stages, possessed inhibitory effects on PC-DTH in mice. To elucidate the potential mechanism of action, we assessed influences of EPZ on activation and functions of T lymphocytes and macrophages as well as inflammation. Results showed that EPZ remarkably attenuated Con A–elicited proliferation and IL-2 production of murine splenocytes in vitro.. Concurrently, EPZ significantly alleviated the ear swelling caused by application of xylene in mice. At examined concentrations, EPZ did not affect the production of nitric oxide from peritoneal macrophages of mice activated with LPS. These findings suggest that inhibition of PC-DTH by EPZ is probably associated with preventive effects on proliferation and differentiation of T lymphocytes during the sensitization stage and anti-inflammatory activities during the effector stage, but not with effects on activation of macrophages and consequent release of nitric oxide.
Recently, mast cells were reported to play an important role in the process of T cell–mediated DTH reactions. Activated mast cells are capable of producing large amounts of tumor necrosis factor-α (TNF-α) and CXC chemokines, macrophage inflammatory protein-1 (MIP-1), α/β, and MIP-2, which enhance DTH reactions by regulating the recruitment of polymorphonuclear leukocytes into sites of inflammation during the effector stage of DTH (Biedermann et al., Citation2000; von Stebut et al., Citation2003). Our previous studies have confirmed that EPZ is able to prevent mast cell activation caused by compound 48/80 and consequent mediator release via. increasing intracellular cAMP content (Dai et al., Citation2004). It is, therefore, postulated that restriction of mast cell activation may contribute to inhibition of DTH by EPZ. Further investigations are needed to ascertain the active compounds of EPZ and the precise mechanism.
References
- Ahmad I, Mehmood Z, Mohammad F, Ahmad S (2000): Antimicrobial potency and synergistic activity of five traditionally used Indian medicinal plants. J Med Aromatic Plant Sci 22–23: 173–176.
- Biedermann T, Kneilling M, Mailhammer R, Maier K, Sander CA, Kollias G, Kunkel SL, Hultner L, Rocken M (2000): Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med 192: 1441–1452. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Dai Y, Hou LF, Chan YP, Cheng L, But PP (2004): Inhibition of immediate allergic reactions by ethanol extract from Plumbago zeylanica stems. Biol Pharm Bull 27: 29–32. [CSA], [CROSSREF]
- Du ZY, Li XY (1998): Cytokine and nitric oxide production by rat microglia stimulated with lipopolysaccharides in vitro.. Acta Pharmacol Sin 19: 257–260.
- Jiangsu New Medical College (1979): Zhongyao Dictionary (Encyclopedia of Chinese Materia Medica). Shanghai, Shanghai Scientific & Technological Press, pp. 711–712.
- Lombardi P, Fournier M, Bernier J, Mansour S, Neveu P, Krzystyniak K (1991): Evaluation of the immunomodulatory potential of diethyl dithiocarbamate derivatives. Int J Immunopharmacol 13: 1073–1084. [PUBMED], [INFOTRIEVE], [CSA]
- MacMicking J, Xie QW, Nathan C (1997): Nitric oxide and macrophage function. Annu Rev Immunol 15: 323–350. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mehmood Z, Ahmad I, Mohammad F, Ahmad S (1999): Indian medicinal plants: A potential source for anticandidal drugs. Pharm Biol 37: 237–242.
- Olagunju JA, Jobi AA, Oyedapo OO (1999): An investigation into the biochemical basis of the observed hyperglycaemia in rats treated with ethanol root extract of Plumbago zeylanica.. Phytother Res 13: 346–348. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Oyedapo OO (1996): Studies on bioactivity of the root extract of Plumbago zeylanica.. Int J Pharmacog 34: 365–369.
- Sharma I, Gusain D, Dixit VP (1991): Hypolipidaemic and antiatherosclerotic effects of plumbagin in rabbits. Indian J Physiol Pharmacol 35: 10–14. [PUBMED], [INFOTRIEVE]
- Simonsen HT, Nordskjold JB, Smitt UW, Nyman U, Palpu P, Joshi P, Varughese G. (2001): In vitro. screening of Indian medicinal plants for antiplasmodial activity. J Ethnopharmacol 74: 195–204. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Terawaki K, Nose M, Ogihara Y (1997): The effects of crude polysaccharide fractions of 4 kinds of kampo-hozai administered orally on nitric oxide production by murine peritoneal macrophages. Biol Pharm Bull 20: 809–811. [PUBMED], [INFOTRIEVE], [CSA]
- von Stebut E, Metz M, Milon G, Knop J, Maurer M (2003): Early macrophage influx to sites of cutaneous granuloma formation is dependent on MIP-1 alpha/beta released from neutrophils recruited by mast cell-derived TNFalpha. Blood 101: 210–215. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Zinyama RB, Bancroft GJ, Sigola LB (2001): Adrenaline suppression of the macrophage nitric oxide response to lipopolysaccharide is associated with differential regulation of tumour necrosis factor-alpha and interleukin-10. Immunology 104: 439–446. [PUBMED], [INFOTRIEVE], [CROSSREF]