Abstract
The effect of Gmelina arborea Roxb. (Verbenaceae) bark and fruit aqueous extracts on paraquat- and hydrogen peroxide–induced oxidative stress was investigated using liver slice culture. Both paraquat and hydrogen peroxide were found to be cytotoxic as measured by release of lactate dehydrogenase from liver slice culture. Addition of bark and fruit extracts along with these cytotoxic agents led to a decrease in lactate dehydrogenase release. Activities of three antioxidant enzymes, namely super-oxide dismutase, catalase, and glutathione reductase, were found to increase on treatment with these pro-oxidants. Addition of the plant extracts along with the pro-oxidants suppressed the enzyme activities. The extracts also displayed antioxidant activity in in vitro radical scavenging assays. Results indicate that Gmelina bark and fruit extracts protected liver slice culture cells by alleviating oxidative stress–induced damage to liver cells.
Introduction
Both paraquat and hydrogen peroxide are known to generate oxidative stress. Hydrogen peroxide (H2O2) itself is a reactive oxygen species (ROS), and paraquat generates superoxide radical by accepting an electron from the mitochondrial electron transport and leads to formation of other ROS (Herai Kei-Jehi et al., Citation1999; Suntres, Citation2002). H2O2 is a ROS encountered by the cell externally and internally on diffusion, whereas paraquat generates ROS intracellularly. Cells are well protected by many nonenzymatic antioxidant substrates as well as enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (Bandyopadhyay et al., Citation1999). An imbalance between the antioxidant defense system and generation of ROS leads to an impairment of cell homeostasis and causes cell toxicity (Berg et al., Citation2004; Devasagayam et al., Citation2004). Antioxidants added externally to cell systems help in mitigating the effect of oxidative stress (Vitaglione et al., Citation2004).
Gmelina arborea. Roxb. (Verbenaceae) is a tree species found in moist deciduous forests of southern and southeastern Asia. It has been introduced as a plantation tree species in Africa and South America primarily for its timber yielding quality (Dvorak, Citation2004). The tree is also valued for its medicinal properties. Almost all parts of the tree are used in folk medicine for treating various stomach disorders, skin problems, and fevers. The chemical constituents of this plant include a variety of flavonoids, iridoid glycosides, and lignans (Anjaneyulu et al., Citation1977; Hosny et al., Citation1998). The crude extract from leaves was observed to have antioxidant activity in vitro. (Naik et al., Citation2003).
Medicinal properties of crude extracts of Gmelina arborea. have been investigated using model animal systems. The extracts are reported to have anti-inflammatory activity (Barik et al., Citation1992), wound-healing properties (Shirwaikar et al., Citation2003), and are known to inhibit platelet aggregation (Faiza & Darakhshanda, Citation1998).
In this study, the aqueous extracts of the bark and fruit of Gmelina arborea. have been used to study their hepatoprotectant and antioxidant activity using liver slice culture. Oxidative stress was generated by H2O2 and paraquat. Liver slice culture offers a very good model compared with isolated hepatocytes as it retains tissue organization and cell-to-cell matrix interactions (Gandolfi et al., Citation1996; George et al., Citation1996; Groneberg et al., Citation2002). This model has been successfully used to assess the effect of several cytotoxic agents such as paracetamol (Lohman et al., Citation1984), aflatoxin B (Uwaifo, Citation1984), paraquat (Togashi et al., Citation1991), and so forth, as well as for checking hepatoprotective activity of compounds such as curcumin (Naik et al., Citation2004).
Materials and Methods
Biologicals
Adult Swiss albino mice (6 to 8 weeks old) of either sex bred in the animal house of the Department of Zoology, University of Pune, were used for the preparation of liver slices. Prior approval for the protocols used involving animals during this work was obtained from the Pune University Institutional Animal Ethical Committee.
Chemicals
All common chemicals used were from one of the following suppliers: SRL (Mumbai, India), BDH (India), Hi-media (Mumbai, India), Sigma-Aldrich (Bangalore, India), or Merck (Mumbai, India).
Preparation of bark and fruit extracts
The bark and the fruits of Gmelina arborea. were obtained from the botanical garden of the Department of Botany, University of Pune. It was authenticated by the Division of Plant Sciences, Agharkar Research Institute, Pune, where a voucher specimen is deposited. Both the bark and fruits were sun-dried, finely powered, and 10% aqueous extracts were prepared as follows. One gram of powder was added to 10 ml of water and homogenized well using a mortar and pestle. The suspension obtained was then centrifuged at 10,000 rpm for 15 min at 4°C. Supernatant was collected and stored in aliquots at − 20°C until use.
Liver slice culture in vitro.
Liver slice culture was maintained following the protocol developed by Wormser et al. (Citation1990) and Invittox Protocol No. 42 (Citation1992). The mice were dissected open after cervical dislocation, and liver lobes were removed and transferred to prewarmed Kreb's Ringer Hepes (KRH) (2.5 mM Hepes, pH 7.4, 118 mM NaCl, 2.85 mM KCl, 2.5 mM CaCl2, 1.5 mM KH2PO4, 1.18 mM MgSO4, 5 mM β-hydroxy butarate, and 4.0 mM glucose). Liver was then cut into thin slices using sharp scalpel blades; the slices, weighing between 4 and 6 mg, were used for the experiment. Each experimental system contained 20–22 slices. These slices were washed with 10 ml KRH medium every 10 min over a period of 1 h. These were then preincubated for 60 min in small plugged beakers containing 2 ml KRH on a shaker water bath at 37°C. At the end of preincubation, the medium was replaced by 2 ml of fresh KRH and incubated for 2 h at 37°C with paraquat (5 mM) or H2O2 (10 mM). At the end of incubation, each group of slices was homogenized in an appropriate volume of chilled potassium phosphate buffer (100 mM, pH 7.8) in an ice bath to give a tissue concentration of 100 mg/ml. The culture medium was collected and used for estimation of lactate dehydrogenase (LDH), which was used as a cytotoxicity marker. The homogenates were centrifuged at 10,000 rpm for 10 min at 4°C and the supernatants assayed for LDH, catalase, superoxide dismutase, and glutathione reductase (GR).
Measurement of LDH activity
Lactate dehydrogenase (LDH; EC 1.1.1.27) was estimated by the method of Wahlefeld (Citation1983). Each unit of enzyme was calculated as 1 µmol of nicotinamide adenine dinucleotide (NAD) reduced per minute. Enzyme units in the medium and in tissue homogenate were estimated and percent release of enzyme from liver slices was calculated as the ratio of LDH activity found in the supernatant to the total LDH (supernatant + homogenate) activity (Wormser & Ben Zakine, Citation1990).
Measurement of antioxidant enzymes
Superoxide dismutase (SOD; EC 1.15.1.1) was assayed spectrophotometrically according to Beauchamp and Fridovich (Citation1971). The extent to which the enzyme decreases the reduction of nitroblue tetrazolium (NBT) by superoxide radical generated by riboflavin in the presence of light was monitored at 560 nm. One unit of enzyme was defined as the amount of enzyme causing 50% reduction in formazan formation under specified conditions. Catalase (CAT; EC 1.11.1.6) assay was carried out according to the method of Aebi (Citation1983). One unit was defined as that amount of the enzyme that converts 1 µmol H2O2 to water in 1 min. Glutathione reductase (GR; EC 1.6.4.2) activity was determined by the protocol of Goldberg (Citation1983). One unit was defined as that amount of the enzyme required to oxidize 1 µmolof Nicotinamide adenine dinucleotide phosphate (NADPH) reduced to Nicotinamide adenine dinucleotide phosphate (NADP) per minute.
Measurement of antioxidant activity of the extracts
Both bark and fruit aqueous extracts were checked for their radical scavenging activities by ferric reducing antioxidant power (FRAP) (Benzie & Strain, Citation1996; Pulido et al., Citation2000), 1,1′-diphenyl-2-picrylhydrazyl (DPPH) (Aquino et al., Citation2001), and ferrylmyoglobin/ABTS (2,2′-azobis-3-ethylbenzthiazoline-6-sulfonic acid) (Alzoreky & Nakahara, Citation2001) assays. One percent, 5%, and 10% concentrations of aqueous extracts were used. The results were expressed as ascorbic acid equivalent antioxidant capacity (AEAC).
In the DPPH radical scavenging assay, a commercially available and stable free radical (DPPH˙), which is soluble in methanol, was used. In its radical form, DPPH˙ has an absorption band at 515 nm, which disappears on reduction by an antioxidant compound. An aliquot (37.5 µl) of the extract was added to 1.5 ml of freshly prepared DPPH˙ solution (0.025 g/l in methanol). Absorbance was measured at 515 nm 20 min after the reaction was started. The calibration curve was plotted with % DPPH˙SCAVENGED versus concentration of the standard antioxidants (L-ascorbic acid).
In the ABTS˙+ radical scavenging assay, the scavenging activities of extracts were determined by using the ferrylmyoglobin/ABTS˙+protocol. The reaction mixture (total volume 2 ml) contained the following substances (final concentrations in the reaction mixture): 2,2-azobis-3-ethylbenzthiozoline-6-sulphonic acid (ABTS) (150 µM), MbIII (2.5 µM), 16.8 µl of the sample, and 978 µl Phosphate buffered saline (PBS). The reaction was initiated by adding 75 µM H2O2 (330 µl), and the lag time in seconds, before absorbance of ABTS˙+ at 734 nm began to increase, was recorded. The calibration curve was plotted with lag time in seconds versus concentration of the standard antioxidants (L-ascorbic acid).
The ferric reducing ability was measured by FRAP assay at low pH. The stock solutions of 10 mM 2,4,6-tripyridyl-S-triazine (TPTZ) in 40 mM HCl, 20 mM FeCl3 · 6H2O, and 0.3 M acetate buffer (pH 3.6) were prepared. The FRAP reagent contained 2.5 ml TPTZ solution, 2.5 ml ferric chloride solution, and 25 ml acetate buffer. It was prepared fresh and warmed to 37°C. Then, 900 µl of FRAP reagent was mixed with 90 µl of distilled water (D/W) and 30 µl of test sample/methanol/DW/standard solutions. The reaction mixture was then incubated at 37°C for 30 min and absorbance was recorded at 595 nm. The concentration of FeSO4 was in turn plotted against concentrations of the standard antioxidant (L-ascorbic acid).
Statistical analyses
Data were analyzed using one-way ANOVA (MINITAB version 13.32). All treatments were compared with each other using Tukey's pairwise comparison.
Results
Release of LDH in liver slice culture
Release of LDH was used as a marker of cytotoxicity in liver slice culture. Release of LDH was six-times more in H2O2 () and paraquat () treated tissues compared with control. When the bark and fruit aqueous extracts were added to the culture along with these two cytotoxic agents, the amount of LDH released was reduced significantly in a concentration-dependent manner (Tables and ). Ascorbic acid was used as a standard antioxidant and protected liver slices from cytotoxic effect of both pro-oxidants used (Tables and ).
Table 1 Effect of Gmelina. bark and fruit extracts in protecting liver cells from H2O2-induced cytotoxicity by ameliorating oxidative stress
Table 2 Effect of Gmelina. bark and fruit extracts in protecting liver cells from paraquat-induced cytotoxicity by ameliorating oxidative stress
Antioxidant enzyme activities in liver slice culture
Both paraquat and H2O2 induce oxidative stress in the cells by generation of ROS. Antioxidant enzymes are known to be induced in response to ROS and play a role in detoxifying these ROS (Eysseric et al., Citation2000). In the case of liver slices treated with paraquat and H2O2, the amount of all three antioxidant enzymes, namely, SOD, CAT, and GR, were found to be increased significantly (Tables and ). In case of SOD, the activity increased 2.5-times when the slices were treated with either paraquat or H2O2. Catalase showed 5-times more activity in case of the slices treated with H2O2 and 2.5-times more activity when treated with paraquat. Glutathione reductase activity increased twofold in the case of the slices treated with paraquat and increased 2.5-times when the slices were treated with H2O2. When both fruit and bark aqueous extracts were added along with the toxicants in the medium, the activity of all three antioxidant enzymes were reduced substantially and were similar to that of untreated cells, especially at the higher concentration used. Ascorbic acid, used as control, also showed reduced antioxidant enzyme activities when added along with the toxicants to the culture.
Antioxidant activity of aqueous fruit and bark extracts
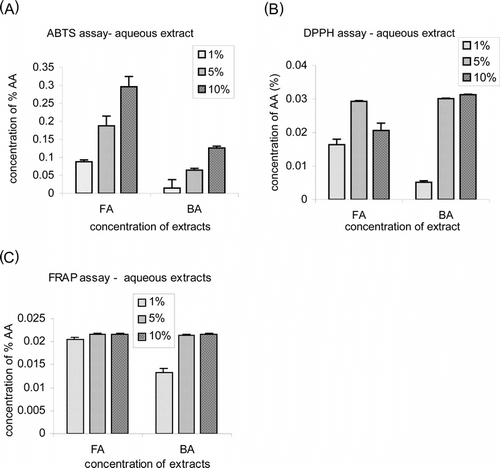
Three different assays were performed to check the antioxidant activities of the extracts used. Both the bark and fruit extracts showed inhibition of ABTS˙+ radical formation in a concentration-dependent manner in the ferrylmyoglobin/ABTS assay (; 0.68 mM and 1.7 mM of ascorbic acid equivalent, respectively). The DPPH assay, in which primary radical scavenging is measured, also revealed potent activity () for both the extracts (1.7 mM and 0.9 mM of ascorbic acid equivalent, respectively). In the FRAP assay, which estimates the capacity to inhibit radical formation, both extracts showed a similar amount of reducing activity (1.23 mM of ascorbic acid equivalent) () at all concentrations.
Figure 1 Antioxidant capacity of Gmelina arborea. fruit (FA) and bark (BA) aqueous extracts estimated by (A) ABTS, (B) DPPH, and (C) FRAP assays. Values are mean ± SE of five different estimations. The antioxidant activity is expressed as equivalent of concentration of water-soluble standard antioxidant ascorbic acid
Discussion
Many herbal extracts have been tested over the years for their medicinal properties such as anti-inflammatory, anticancer, antidiabetic, and antioxidant activities in vivo. and in vitro.. Several cell culture systems provide valuable in vitro. approaches for screening of plant extracts for their potential therapeutic properties as well as for the elucidation of possible mechanisms of action at the molecular level (Gebhardt, Citation2000)
In these experiments, we have studied the hepatoprotectant and antioxidative activity of aqueous extracts of Gmelina arborea. bark and fruit. Although anti-inflamotory and wound-healing properties of Gmelina arborea. extracts have been studied (Barik et al., Citation1992; Shirwaikar et al., Citation2003), the antioxidant and hepatoprotective properties have not been reported in animal systems. Oxidative stress was induced by adding paraquat and H2O2 to liver slice culture. Such a culture offers a very good test system as it provides desirable complexity of structurally and functionally intact cells. It retains intact tissue architecture and more closely mimics the situation in vivo. as compared with isolated hepatocytes.
Both paraquat and H2O2 induce oxidative stress in the cell, and damage caused due to it can be reduced by adding antioxidants from plant extracts (Tseng et al., Citation1995; Wei et al., Citation2000; Jand & Surh, Citation2001). Our studies showed that both the pro-oxidants used were highly toxic to the cells as seen by the increase in LDH release by treated cells. On application of the Gmelina arborea. extracts, the cytotoxic effect was substantially lowered probably through the reduction of oxidative stress. Activities of three antioxidant enzymes were measured to assess the oxidative stress in the cells. Oxidative SOD and CAT are known to prevent damage by directly scavenging the harmful active oxygen species like superoxide and H2O2, respectively. Glutathione reductase plays a role in recycling the oxidized glutathione to reduced glutathione, which acts as an antioxidant. It has been demonstrated that the paraquat-induced oxidative stress leads to dysfunctioning of glutathione redox cycle in endothelial cells (Tukamoto et al., Citation2002).
We found that the activities of SOD, CAT, and GR significantly increased in cultures treated with paraquat and H2O2, indicating that the toxicity of these pro-oxidants led to an oxidative stress in liver tissue and induced the activity of these antioxidant enzymes. Earlier studies have shown that these pro-oxidants induce generation of lipid hydroperoxides of rat liver slices (Togashi et al., Citation1991). When the Gmelina arborea. extracts were added along with these pro-oxidants, the activity of all three enzymes decreased to levels comparable with those seen either in untreated cultures or in cultures treated with the known antioxidant. Our results indicated that Gmelina arborea. extracts protected the liver tissue against the oxidative stress and hence did not lead to induction of antioxidant enzymes. The antioxidant properties of Gmelina arborea. were also demonstrated using in vitro. radical scavenging assays (DPPH, FRAP, ABTS).
In conclusion, our studies show that hepatotoxicity caused by oxidative stress generated by the pro-oxidants paraquat and H2O2 could be prevented by the antioxidative properties shown by Gmelina arborea. extracts suggesting a possible therapeutic role for this plant.
Acknowledgments
The authors acknowledge financial support from the University Grants Commission, New Delhi, under the Departmental Special Assistance (DSA) program. Priyanjali Dixit is a recipient of a fellowship from the University of Pune-BARC Joint Research Programme.
References
- Aebi HE (1983): Catalase. In: Bermeyer HU, ed., Methods of Enzymatic Analysis, Vol. 3. Weinhein, Verlagchemie GmbH, pp. 277–282.
- Alzoreky N, Nakahara K (2001): Antioxidant activity of some edible Yemeni plants evaluated by ferrylmyoglobin/ABTS˙+ assay. Food Sci Technol Res 7: 141–144. [CSA]
- Anjaneyulu, ASR, Madhusudhana Rao A, Kameswara Rao V, Ramchandra Row L (1977): Novel hydroxy lignans from the heartwood of Gmelina arborea.. Tetrahedron 33: 133–143. [CROSSREF], [CSA]
- Aquino R, Moreli S, Lauro MR, Abdo S, Saija A, Tomain A (2001): Phenolic constituents and antioxidant activity of an extract of Anthurium versicolor. leaves. J Nat Prod 64: 1919–1923. [CROSSREF], [CSA]
- Bandyopadhyay U, Das D, Banerjee RK (1999): Reactive oxygen species: Oxidative damage and pathogenesis. Curr Sci 77: 658–666. [CSA]
- Barik BR, Bhaumik T, Patra A, Roy S, Susan T, Alan M, Kundu AB, Chatterjee A (1992): Premnazole, an isoxazole alkloid of Premna integrofolia. and Gmelina arborea. with antiinflammatory activity. Fitoterapia 63: 295–299. [CSA]
- Beauchamp C, Fridovich I (1971): Superoxide dismutase; improved assay and an assay applicable to acrylamide gel. Anal Biochem 44: 276–287. [INFOTRIEVE], [CROSSREF], [CSA]
- Benzie IFF, Strain JJ (1996): The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power. The FRAP assay. Anal Biochem 239: 70–76. [INFOTRIEVE], [CROSSREF], [CSA]
- Berg D, Youdim MB, Riederer P (2004): Redox imbalance. Cell Tissue Res 318: 201–213. [INFOTRIEVE], [CROSSREF], [CSA]
- Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD (2004): Free radicals and antioxidants in human health: Current status and future prospects. J Assoc Physicians India 52: 794–804. [INFOTRIEVE], [CSA]
- Dvorak WS (2004): World view of Gmelina arborea.! Opportunities and challenges. New Forests 28: 111–126. [CROSSREF], [CSA]
- Eysseric H, Gonthier B, Soubeyran A, Richard MJ, Daveloose D, Barret L (2000): Effects of chronic ethanol exposure on acetaldehyde and free radical production by astrocytes in culture. Alcohol 21: 117–125. [INFOTRIEVE], [CROSSREF], [CSA]
- Faiza H, Darakhshanda S (1998): The inhibition of platelet aggregation and the related physiological responses with the crude drug extract of Gmelina arborea.. In: Sixth International Symposium on New Trends in Natural Products Chemistry Publication, Karachi, Pakistan, pp. 279–286.
- Gandolfi AJ, Wijeweera J, Brendel K (1996): Use of precision-cut liver slices as an in vitro. tool for evaluating liver function. Toxicol Pathol 24: 58–61. [INFOTRIEVE], [CSA]
- Gebhardt R (2000): In vitro. screening of plant extracts and phytopharmaceuticals: Novel approaches for the elucidation of active compounds and their mechanisms. Planta Med 66: 99–105. [INFOTRIEVE], [CROSSREF], [CSA]
- George E, Hamilton G, Westmoreland C (1996): The use of in vitro. model in hepatotoxicity testing. Toxicol Ecotoxicol News 3: 142–151. [CSA]
- Groneberg DA, Grosse-Siestrup C, Fischer A (2002): In vitro. models to study hepatotoxicity. Toxicol Pathol 30: 394–399. [INFOTRIEVE], [CROSSREF], [CSA]
- Goldberg DM (1983): Glutathione reductase. In: Bermeyer HU, ed., Methods of Enzymatic Analysis, Vol. 3. Weinhein, Verlagchemie GmbH, pp. 277–282.
- Herai Kei-Jehi, Pan J, Shimada H, Izuhara T, Kurihara T, Moriguchi K (1999): Cytochemical energy-filtering transmission electron microscopy of mitochondrial free radical formation in paraquat cytotoxicity. J Electron Microsc (Tokyo) 48: 289–296. [CSA]
- Hosny M, Rosaazza JPN, Gmelinosides AL (1998): Twelve acylated iridoid glycosides from Gmelina arborea.. J Nat Prod 61: 734–742. [INFOTRIEVE], [CROSSREF], [CSA]
- Invittox Protocol No. 42, (1992): Liver slice hepatotoxicity screening system. The ERGATT/FRAME Data Bank of in vitro. Techniques in Toxicology. Nottingham England Invittox.
- Jand JH, Surh YJ (2001): Protective effects of resveratrol on hydrogen peroxide-induced apoptosis in rat pheochromocytoma (PC-12) cells. Mutat Res 496: 181–190. [CSA]
- Lohman J, Lessing U, Schriewer H, Clemen M, Gerlach U (1984): The influence of paracetamol on the hepatic biosynthesis of lecithin. Arch Toxicol (Suppl) 7: 236–739. [CSA]
- Naik D, Joshi JP, Kamat JP, Chintalwar GJ, Chattopadhaya S (2003): Identification and purification of flavonoids from Gmelina arborea. root with antioxidant potential. In: National Symposium on Radiation and Photochemistry. Indian Society for Radiation and Photochemical Sciences, Abstract no. R 23.
- Naik RS, Mujumdar AM, Ghaskadbi S (2004): Protection of liver cells from ethanol cytotoxicity by curcumin in liver slice culture in vitro.. J Ethnopharmacol 95: 31–37. [INFOTRIEVE], [CROSSREF], [CSA]
- Pulido R, Bravo I, Saura-Calixto F (2000): Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing antioxidant power assay. J Agric Food Chem 48: 33396–33402. [CROSSREF], [CSA]
- Shirwaikar A, Ghosh S, Padma GM Rao (2003): Effects of Gmelina arborea. Roxb leaves on wound healing in rats. J Nat Remedies 3: 45–48. [CSA]
- Suntres ZE (2002): Role of antioxidants in paraquat toxicity. Toxicology 180: 65–77. [INFOTRIEVE], [CROSSREF], [CSA]
- Togashi H, Shinzawa H, Wakabayashi H, Nakamura T, Yong H, Yamada N, Ukai K, Okuyama Y, Takahashi T, Ishikawa M (1991): Superoxide is involved in the pathogenesis of paraquat induced injury in cultured rat liver slices. Hepatology 14: 707–714. [INFOTRIEVE], [CROSSREF], [CSA]
- Tseng HJ, Chu CY, Huang JM, Shiow SJ, Wang CJ (1995): Crocetin protects against oxidative damage in rat primary hepatocytes. Cancer Lett 97: 61–67. [INFOTRIEVE], [CROSSREF], [CSA]
- Tukamoto M, Tampo Y, Sawada M, Yonaha M (2002): Paraquat-induced oxidative stress and dysfunction of the glutathione redox cycle in pulmonary microvascular endothelial cells. Toxicol Appl Pharmacol 178: 82–92. [CROSSREF], [CSA]
- Uwaifo AO (1984): Inhibition of oxygen uptake in liver slices of three mammalian species (rat, rabbit, guinea pig) by aflatoxin B1 (AFB1). Toxicology 31: 33–39. [INFOTRIEVE], [CROSSREF], [CSA]
- Vitaglione P, Morisco F, Caporaso N, Fogliano V (2004): Dietary antioxidant compounds and liver health. Crit Rev Food Sci Nutr 44: 575–586. [INFOTRIEVE], [CSA]
- Wahlefeld AW, (1983): UV-method with L-Lactate and NAD. In: Bermeyer HU, ed., Methods of Enzymatic Analysis, Vol. 3. Weinhein, Verlagchemie GmbH, pp. 126–133.
- Wei T, Ni Y, Hou J, Chen C, Zhao B, Xin W (2000): Hydrogen peroxide induced oxidative damage and apopotosis in cerebeller granule cells: protection by Ginkgo biloba. extract. Pharmacol Res 41: 427–433. [INFOTRIEVE], [CROSSREF], [CSA]
- Wormser U, Ben Zakine S (1990): The liver slice system—an in vitro. acute toxicity test for assessment of hepatotoxins and their antidotes. Toxicol In vitro 4: 449–451. [CROSSREF], [CSA]
- Wormser U, Ben Zakine S, Stivelband E, Eisen O, Nyska A (1990): The liver slice system: A rapid in vitro. acute toxicity test for primary screening of hepatotoxic agents. Toxicol In vitro 4: 783–789. [CROSSREF], [CSA]