Abstract
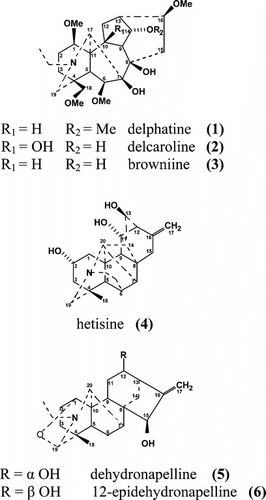
Three norditerpenoid alkaloids, delphatine (1), delcaroline (2), and browniine (3), and three diterpenoid alkaloids, hetisine (4), dehydronapelline (5), and 12-epidehydronapelline (6), have been isolated and identified from the aerial parts of Consolida olopetala. (Boiss.) Hayek (Ranunculaceae).
Introduction
Aconitum, Delphinium., and Consolida. species (Ranunculaceae) are toxic plants due to the diterpenoid alkaloids they contain. These alkaloids are neurotoxic agents, causing bradycardia, muscle system spasms, hypotension, and death by arrest of respiration. Aconitum. preparations have been used as cardiotonics, febrifuges, sedatives, and anodynes. Delphinium. and Consolida. (formerly known as Delphinium.) extracts have been employed in analgesic balms and also as sedatives, emetics, and anthelmintics. They are also known to possess insecticidal and growth-inhibiting activities (Bisset, Citation1981; Benn & Jacyno, Citation1983; Baytop, Citation1984; Ulubelen et al., Citation2001; Gonzalez et al., Citation2005). Testing of pure diterpenoid alkaloids and their derivatives for cardiovascular (bradycardic and hypotensive) action has also been conducted (Chiao et al., Citation1995; Desai et al., Citation1998). In continuation of our investigations of Turkish Aconitum., Delphinium., and Consolida. species for their diterpenoid alkaloid content (Meriçli et al., Citation1997Citation1998Citation1999Citation2000Citation2001), we have now studied Consolida olopetala.. The plant is very rare, grows in Turkey (Gray, Citation1965), and has not previously been studied chemically.
Materials and Methods
General
NMR spectra were recorded on a Bruker 500 MHz spectrometer. Mass spectra (MS) were determined on a Finnigan MAT 90 spectrometer. Vacuum-liquid chromatography (VLC) was carried out with Merck Al2O3 (EM 1085) and SiO2 60 G (7731). Chromatographic separations on a Chromatotron were carried out on rotors coated with a 1-mm-thick layer of Merck Al2O3 60 GF-254 (1092) or SiO2 PF-254 (7749). Thin-layer chromatograms were run using the solvent system toluene:EtOAc:DEA (9:2:1 or 7:2:1) and CHCl3:MeOH:NH4OH (9:1:1 or 9:2:1).
Plant material
Consolida olopetala. (Boiss.) Hayek was collected between Konya and Karaman, Turkey, on the roadsides at an altitude of 1100 m in July 2000 and was identified by one of us (H.O.). A voucher specimen (Ozcelik 8465) is deposited in the herbarium of the Faculty of Science and Literature, Süleyman Demirel University.
Extraction and isolation
Dried and powdered aerial parts of the plant (2.5 kg) were extracted with 90% EtOH by percolation at room temparature and the extract obtained evaporated to dryness in vacuo.. The residue was treated with 0.5 N H2SO4 and extracted with CHCl3. NaOH (5%) was then added to the aqueous solution (cooled in ice) to bring it to pH 10. The solution was again extracted with CHCl3. The CHCl3 extract was evaporated to dryness, yielding 6 g of crude alkaloid mixture.
The crude alkaloid extract (6 g) obtained from 2.5 kg of the aerial parts of C. olopetala. was first separated by VLC on SiO2 column with petroleum ether:CHCI3:MeOH mixtures. VLC fractions 24–27 (CHCI3:MeOH 95:5 to 92:8) (450 mg) were combined and chromatographed on a SiO2 rotor with petroleum ether:CHCI3:MeOH mixtures to give 12-epidehydronapelline (15 mg), delphatine (80 mg), and browniine (7 mg). VLC fraction 28 (CHCI3:MeOH, 92:8) (180 mg) was applied on preparative TLC, and dehydronapelline was isolated (12 mg). VLC fraction 31 (600 mg), (CHCI3:MeOH, 70:3) was chromatographed on a Al2O3 rotor with petroleum ether:CHCI3:MeOH mixtures to give delcaroline (16 mg) and hetisine (16 mg). Thin-layer chromatograms were run using the solvent system toluene:EtOAc:DEA (7:4:1) and CHCI3:MeOH:NH4OH (9:2:1 or 9:1:1).
All these compounds were identified by comparison of their 1H and 13C, Distortionless Enhancement Polarization Transfer (DEPT) NMR data and Co-TLC behavior with those of authentic samples. The 13C NMR data of the isolated compounds are shown in .
Table 1 13C NMR data of Consolida olopetala. alkaloids
Results and Discussion
Three norditerpenoid alkaloids, lycoctonine type, delphatine (1), delcaroline (2), and browniine (3), were isolated from the aerial parts of Consolida olopetala.. Their structures are very similar to each other (). The norditerpenoid alkaloids are very toxic compounds. In addition to these alkaloids, one diterpenoid alkaloid of the atisine type, hetisine (4), and two diterpenoid alkaloids of the veatchine type, dehydronapelline (5) and 12-epidehydronapelline (6), were also isolated. Veatchin-type alkaloids do not always occur in Consolida. species, so this result can be important from the chemotaxonomical point of view. All these results show this rare species contains toxic diterpenoid alkaloids.
Acknowledgment
A.H.M. thanks the Alexander von Humboldt Foundation for a fellowship.
References
- Baytop T (1984): Therapy with Medicinal Plants in Turkey, Istanbul, University of Istanbul Publication No. 3295, pp. 187, 312.
- Benn MH, Jacyno JM (1983): The toxicology and pharmacology of diterpenoid alkaloids. In: Pelletier SW, ed., Alkaloids, Chemical and Biological Perspectives, Vol. 1. New York, Wiley Interscience, pp. 155–210.
- Bisset NG (1981): Arrow poisons in China Part 11, Aconitum.—Botany, chemistry and pharmacology. J Ethnopharmacol 4: 247–336. [INFOTRIEVE], [CSA]
- Chiao H, Pelletier SW, Desai HK, Rabagay NR, Caldwell RW (1995): Effect of diterpenoid alkaloids on cardiac sympathetic efferent and vagal afferent nerve activity. Eur J Pharmacol 283: 103–106. [INFOTRIEVE], [CROSSREF], [CSA]
- Desai HK, Hart BP, Caldwell RW, Huang J, Pelletier, SW (1998): Certain norditerpenoid alkaloids and their cardiovascular action. J Nat Prod 61: 743–748. [INFOTRIEVE], [CROSSREF], [CSA]
- Gonzalez P, Marin C, Rodriguez-Gonzalez I, Hitos AB, Rosales MS, Reina M, Diaz JG, Gonzalez-Coloma A, Sanchez-Moreno M (2005): In vitro. activitiy of C20-diterpenoid alkaloid derivatives in promastigotes and intracellular amastigotes of Leishmania infantum.. Int J Antimicrob Agents 25: 136–141. [INFOTRIEVE], [CROSSREF], [CSA]
- Gray SF (1965): Consolida. (DC). In: Davis PH, ed., Flora of Turkey and the East Aegean Islands, Vol. I. Edinburgh, University Press, pp. 119–134.
- Meriçli AH, Meriçli F, Seyhan V, Ulubelen A, Desai HK, Joshi BS, Teng Q, Pelletier SW (1997): Isolation and structure of raveyine, a novel norditerpenoid alkaloid from Consolida raveyi. (Boiss.) Schröd. Heterocycles 45: 1955–1965. [CSA]
- Meriçli AH, Meriçli F, Ulubelen A, Desai HK, Joshi BS, Pelletier SW, Özden S, Küçükislamoğlu M (1998): Consolarine, a novel norditerpenoid alkaloid from Consolida armeniaca.. Heterocyles 47: 329–335. [CSA]
- Meriçli AH, Meriçli F, Dogru E, Özçelik H, Atta-ur-Rahman, Ulubelen A (1999): Diterpenoid and norditerpenoid alkaloids from Delphinium carduchorum.. Phytochemistry 51: 337–340. [CROSSREF], [CSA]
- Meriçli AH, Meriçli F, Ulubelen A, Bahar M, llarslan R, Algül G, Desai HK, Teng Q, Pelletier SW (2000): Diterpenoid alkaloids from the aerial parts of Aconitum anthora. L. Pharmazie 55: 696–698. [CSA]
- Meriçli AH, Meriçli F, Desai HK, llarslan R, Ulubelen A, Pelletier SW (2001): Diterpenoid alkaloids from Delphinium virgatum. Poiret. Pharmazie 56: 418–419. [CSA]
- Ulubelen A, Meriçli AH, Meriçli F, Kilincer N, Ferizli AG, Emekçi M, Pelletier SW (2001): Insect repellent activity of diterpenoid alkaloids. Phytother Res 15: 170–171. [INFOTRIEVE], [CROSSREF], [CSA]