Abstract
Matrix metalloproteinases (MMPs) play a critical role in several physiologic and pathologic events. There is some evidence that indicates the involvement of MMPs in tumor invasion and inflammatory diseases. Here we study different extracts of Geum kokanicum. Regel & Schmalh. ex Regel (Rosaceae) roots. The potential inhibitory effect of these extracts on the invasive phenotype of Wehi-164 cells, a typical fibrosarcoma cell line, was assessed through a dose-response and time-course fashion using diclofenac sodium as a reference drug. A colorimetric assay was also used to investigate potential cytotoxicity properties of the extracts. The total extract of the roots was found to exhibit a selective inhibitory effect on tumor cell invasion. The polar extracts of G. kokanicum. roots showed potent inhibitory effect on MMPs activity at minimal cytotoxic doses. However, nonpolar extracts of the plant roots did not exhibit this exclusive anti-invasive effect. Taken together, the potent anti-invasive characterisitics of polar G. kokanicum. root extracts might be promising in the preparation of anticancer therapeutic derivatives owing to the fact that inhibition of MMPs activity has been employed in modality therapy in such major pathological processes.
Introduction
Most investigators agree that matrix metalloproteinases (MMPs) are critical enzymes in tumor growth invasion, metastasis (Stetler-Stevenson et al., Citation1996; Fidler, Citation1997; Jones et al., Citation1999), and neovascularization (John & Tuszynski, Citation2001; Nguyen et al., Citation2001). MMPs are a family of highly homologous, zinc- and calcium-dependent endopeptidase that cleave most, if not all, components of the extracellular matrix (ECM). The destruction of the extracellular matrix eventually leads to tumor invasion, metastasis, and angiogenesis (Heath & Grochow, Citation2000). Thus, each component with a potential to inhibit MMP expression, such as a nonsteroidal anti-infalammatory agent like diclofenac sodium, is able to reduce the risk of cancer (Saadat et al., Citation2003).
In our search for anti-invasive natural products, we have selected an extract prepared from the roots of Geum kokanicum. Regel & Schmalh. ex Regel (Rosaceae). G. kokanicum. is distributed in the northern part of Iran in the Esfarayen region. The local people use the roots of G. kokanicum. for the treatment of diarrhea and gastric disorders (Abotorabi, Citation2002). However, no report on the antitumor effect of this genus has been published in the literature. Here we use enzyme zymography to examine the influence of the various extracts of G. kokanicum. roots on the expression of MMPs and their cytotoxic effects on a fibrosarcoma cell line.
Materials and Methods
Plant collection
The roots of Geum kokanicum. were collected from the northern part of Iran's Khorasan Province (2250 m above sea level) and air-dried at room temperature. A sample was authenticated by Dr. G. Amin, and a voucher speciemen was preserved in the herbarium of the Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.
Plant extracts and chemicals
Different extracts were used during this investigation. All experiments were performed based on the dry mass of concentrated extract. Diclofenac sodium was supplied by Daru-Paksh Co. (Tehran, Iran) and used as the positive control in all experiments. The different extracts and the reference drug were dissolved in dimethylsulfoxide and used at different concentrations (0, 10, 20, 40, 80 µg/ml) for further investigation.
Total extract
The roots were dried, chopped (100 g), and macerated with 80% ethanol solution for 3 days with three changes of the solution. The resulting extract was filtered and evaporated into a dried powder extract (6.7 g, 6.7%).
Sequential extracts
Different samples were prepared mixing sequental 500 g extractions of dried and chopped roots of the plant with petroleum ether (A, 2.3 g dry weight corresponding with 0.4%) (bp. 40–60°C), dicholoromethane (B, 1.65 g dry weight corresponding with 0.33%), methanol (C, 49.52 g dry weight corresponding with 9.9%), and water (D, 11.6 g dry weight corresponding with 2.3%), respectively.
Cell culture
The Wehi-164 cells, a typical fibrosarcoma cell line was seeded in 96-well tissue culture plates. Cells were maintained in a RPMI-1640 medium that was supplemented with 5% fetal calf serum, plus antibiotics, at 5% CO2, 37°C, and saturated humidity. The fibrosarcoma Wehi-164 cell line was obtained from the National Cell Bank of Iran (NCBI), Pasteur Institute of Iran, Tehran, Iran.
Dose-response analysis
Triplicate, twofold dilutions of plant extracts and the reference drug were transferred to overnight cultured cells. Nontreated cells were used as controls. Cells were cultured overnight and were then subjected to colorimetric assay. Cytotoxicity was expressed as the percentage of viable cell at different concentrations of samples. IC50 was calculated as the dose at which 50% of the cell death occurred relative to the untreated cells. The corresponding supernatants of the cultured cells were used for zymoanalysis.
Colorimetric assay
In the cytotoxicity assay, cells in the exponential phase of growth were incubated for 24 h at 37°C with 5% CO2 with a serial dilution of extract. Cell proliferation was evaluated by a modified crystal violet colorimetric assay (Saadat et al., Citation2003). After each experiment, the cells were washed with ice-cold PBS and fixed in a 5% formaldehyde solution. Fixed cells were stained with 1% crystal violet. Stained cells were lysed and solubilized with a 33.3% acetic acid solution. The density of developed purple color was read at 580 nm.
Zymoanalysis
This technique has been used for the detection of gelatinase (collagenase type IV or matrix metalloproteinase type 2, MMP-2) and MMP-9 in conditioned media (Heussen & Dowdle Citation1980). Briefly, aliquots of conditioned media were subjected to electrophoresis in a gelatin-containing polyacrylamide gel, in the presence of sodium dodecyl sulfate (SDS) under nonreducing conditions. After electrophoresis, SDS was removed by repeated washing with Triton X100. The gel slabs were then incubated at 37°C overnight in a gelatinase-activating buffer and subsequently stained with Coomassie Brilliant Blue R250 (Sigma Chemical Co., Pool, UK). After intensive destaining, proteolysis areas appeared as clear bands against a blue background. Using a gel documentation system, quantitative evaluation of both the surface and intensity of lysis bands, on the basis of gray levels, were compared relative to nontreated control wells and expressed as a percentage of the “relative expression” of gelatinolytic activity. The IC50 for the MMP inhibitory effect was calculated as doses at which 50% of MMP inhibition occurred relative to untreated control cells.
Statistical analyses
The differences in cell cytotoxicity and gelatinase zymography were compared using the Student's t.-test. p values <0.05 were considered significant.
Results and Discussion
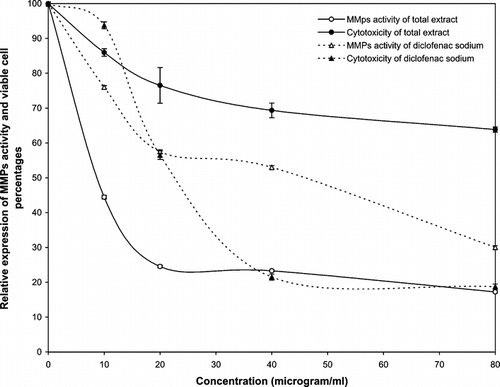
The cytotoxicity of the different extracts of the G. kokanicum. roots and the reference drug were evaluated in vitro. against the fibrosarcoma cell line (Wehi-164) at 10, 20, 40, and 80 µg/ml. Our cytotoxicity analysis of the total extract shows a direct dose-response relationship with the total extract of G. kokanicum.; cytotoxicity increased at higher concentrations (). Cell cytotoxicity of diclofenac sodium versus the total extract of G. kokanicum. is also illustrated in . The presence of 80 µg/ml of G. kokanicum. total extract moderately inhibited the growth of the cell line, whereas lower dose levels (10 µg/ml) showed a negligible cytotoxicity with a viability percentage of more than 85%. Diclofenac sodium showed increasing MMPs inhibitory potentials >10 µg/ml, with strong cytotoxicity from 20 to 80 µg/ml. Conversely, the comparison of the cytotoxic effect of the total extract of the G. kokanicum. root with the reference drug revealed that the former had lower toxicity. Subsequently, to be devoid of nonselective activity, the anti-invasive property of the roots of G. kokanicum. was specifically investigated at minimal toxic dose levels of 10 and 40 µg/ml. The inhibition of the total extract of G. kokanicum. roots on the invasion of the Wehi-164 cells through the matrix membrane are also presented in . As shown in , the invasion of Wehi-164 cells was greatly inhibited at concentrations above 20 µg/ml. At 10 µg/ml, the G. kokanicum. roots were able to inhibit the invasion by more than 50% with less cytotoxicity.
Based on our zymographic analysis of diclofenac sodium, the reduction of MMP expression was associated with increased concentrations of this drug. This result is in accordance with the results of previous studies (Sadowski & Steinmeyer Citation2001; Saadat et al., Citation2003). However, this drug is too toxic to use at high doses, as the reduction of MMP is mostly associated with total cell death. The total extract of the plant did not show high toxicity at all tested concentrations but demonstrated significant inhibition of MMP activity in dose-response fashion. The IC50 values for the total extract and the reference drug (the concentration that inhibits the invasion of the Wehi-164 cells through the matrix membrane by 50% relative to untreated control) were calculated as 8.5 and 47 µg/ml, respectively.
Further to the results discussed above, other different sequential extracts that were tested for cytotoxicity against Wehi-164 cells showed dose-dependent responses. Nonpolar extracts (A, B), as well as diclofenac sodium, showed great cytotoxicity against the cell line used, whereas polar extracts (C, D) indicated moderate toxicity at certain concentrations ( and ). The IC50 values for cytotoxicity assay of the cell line are reported in . In the invasive assay, the petroleum ether and dichloromethane extracts (A, B) exhibited a gradual decrease in MMPs activity at lower concentrations (< 20 µg/ml). In contrast, the invasion of Wehi-164 cells was significantly inhibited at lower concentrations of methanol and water extracts. The inhibitory effects of samples that received a dose of 10 µg/ml were about 45% methanol and 53% water. This activity could be observed for petroleum ether and dichloromethane extracts at higher concentrations (>40 µg/ml).
Figure 2 Cytototoxic and MMPs analysis of petroleum ether (A) and dichloromethane (B) extracts of Geum kokanicum. roots
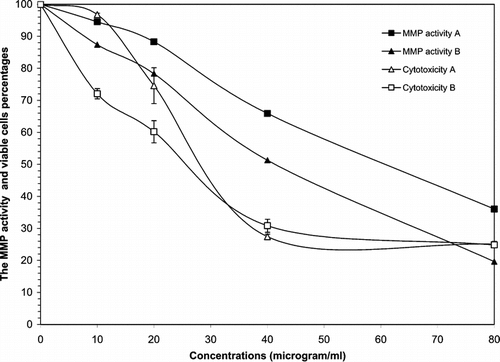
Figure 3 Cytototoxic and MMPs analysis of methanol (C) and water (D) extracts of Geum kokanicum. roots
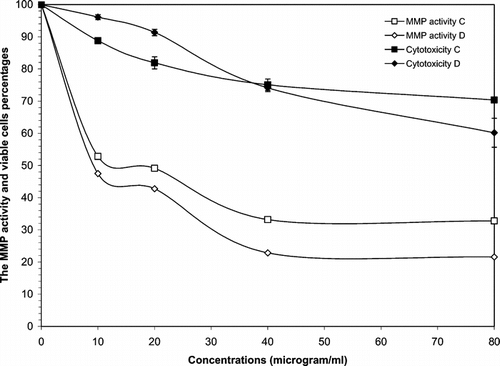
Table 1 IC50 values for cytotoxicity and matrix metalloproteinases (MMPs) activities of different Geum kokanicum. root extracts obtained from cytotoxicity assay and zymoanalysis data
In conclusion, we demonstrated that polar extracts of G. kokanicum. roots show minimal cytotoxic effects, especially at low doses, and maintain an inhibitory influence on MMPs activity as compared with the reference drug and nonpolar extracts that were prepared by sequentional extraction of the roots. Regarding their chemical composition, the G. kokanicum. roots might contain various polar compounds that are extractable with polar solvents. According to the critical role of MMPs in many pathological disorders, this potent extract represents a promising approach to the treatment of a variety of malignant and inflammatory disorders. Future work, however, should focus on the purification and identification of active compound(s) to gain a better perspective of its properties.
Acknowledgment
This research was supported by a grant from the Vice Chancellor for Research, Tehran University of Medical Sciences, Tehran, Iran. We also appreciate scientific assistance from Tehran University Working Group on Natural Products and Medicinal Plants (TUNPMP).
References
- Abotorabi H (2002): The ethnobotany and phytochemistry of plant distributed in the northern part of Iran in the Roeen and Esfarayen regions. Pharm.D. thesis, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.
- Fidler IJ (1997): Molecular biology of cancer: Invasion and metastasis. In: DeVita VT, Jr, Hellman S, Rosenberg SA, eds., Cancer Principles and Practice of Oncology. Philadelphia, Lippincott–Raven, pp. 135–152.
- Heath EI, Grochow LB (2000): Clinical potential of matrix metalloprotease in cancer therapy. Drugs 59: 1043–1055. [INFOTRIEVE], [CSA]
- Heussen C, Dowdle EB (1980): Electrophoretic analysis of plasminogen activator in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Chem 102: 196–202. [CSA]
- John A, Tuszynski G (2001): The role of matrix metalloproteinase in tumor angiogenesis and tumor metastasis. Pathol Oncol Res 7: 14–23. [INFOTRIEVE], [CSA]
- Jones L, Ghaneh P, Humphreys M, Neoptolemos JP (1999): The matrix metalloproteinases and their inhibitors in the treatment of pancreatic cancer. Ann NY Acad Sci 880: 288–307. [INFOTRIEVE], [CROSSREF], [CSA]
- Nguyen M, Arkell J, Jackson CJ (2001): Human endothelial gelatinases and angiogenesis. Int J Biochem Cell Biol 33: 960–970. [INFOTRIEVE], [CROSSREF], [CSA]
- Saadat F, Zomorodian K, Pezeshki M, Khorramizadeh MR (2003): The potential role of nonsteroidal anti-inflammatory drugs (NSAIDS) in chemopreventation of cancer. Pak J Med Sci 19: 13–17. [CSA]
- Sadowski T, Steinmeyer J (2001): Effects of tetracyclines on the production of matrix metalloproteinases and plasminogen activators as well as of their natural inhibitors, tissue inhibitor of metalloproteinases-1 and plasminogen activator inhibitor-1. Inflamm Res 50: 175–182. [INFOTRIEVE], [CROSSREF], [CSA]
- Stetler-Stevenson WG, Hewitt R, Corcoran M (1996): Matrix metalloproteinases and tumor invasion: From correlation and causality to the clinic. Semin Cancer Biol 7: 147–154. [INFOTRIEVE], [CROSSREF], [CSA]