Abstract
Matrine is an alkaloid isolated from the medicinal plant Sophora flavescens Ait (Leguminosae) with various pharmacological and physiological effects. Here, we investigate its combined effects with imatinib, a specific tyrosine kinase inhibitor used clinically against leukemia cells, on bcr/abl-positive leukemia K562 cells. The results indicate that matrine enhanced imatinib-induced apoptosis in K562 cells through downregulation of protein levels of bcr/abl and phospho-Erk1/2.
Introduction
Sophora flavescens. Ait (Leguminosae) (SF) is the dry root of a plant growing in China, Japan, and some European countries. It is commonly used in traditional Chinese medicine to treat a range of diseases, including cancer, viral hepatitis, cardiac arrhythmia, and skin diseases (Niu, Citation1997). Matrine (C15H24N2O, MW = 248.36) is one of the major alkaloid components in this herb and it plays a major role in various functions of SF. Matrine injection has been used to treat hepatitis in China (Cai et al., Citation1997). Previous studies have shown that matrine possesses activities against leukemia cells, and the effects are associated with alteration of protein tyrosine phosphorylation (Zhang et al., Citation2001; Liu et al., Citation2005).
In clinics, the bcr/abl fusion protein is a hallmark of nearly all cases of chronic myelogenous leukemia (CML). It is derived from the Philadelphia chromosome (Ph +), which is formed by a reciprocal translocation between chromosome 9 and 22 (Nowell et al., Citation1960). The bcr/abl oncogene encodes a chimeric protein with constitutive tyrosine kinase activity (Dhut et al., Citation1990). This deregulated activity is believed to be the pathogenic origin of CML: the signal pathways activated by bcr/abl are considered to cause cell transformation and leukemogenesis through promoting proliferation and inhibiting apoptosis (Daley et al., Citation1990; Lugo et al., Citation1990). Imatinib, a specific inhibitor of tyrosine kinases, has remarkable successes in the treatment of CML (Druker et al., Citation1996; Kantarjian et al., Citation2002). However, like other antileukemia drugs, imatinib resistance has been reported in some CML patients, and low efficacy has been found in the blast crisis of CML (Gorre et al., Citation2001; Roumiantsev et al., Citation2002). Lines of evidence indicate that combinations of imatinib with other antileukemia agents has synergetic or additive effects in apoptosis induction in K562 cells (Kano et al., Citation2001; Thiesing et al., Citation2001).
In this study, we investigate the effects of matrine on imatinib-induced apoptosis in bcr/abl-positive K562 cells. To obtain insights into the interactive effects of matrine in combination with imatinib against K562 leukemia cell line, we compared protein levels of the downstream signal pathways mediated by bcr/abl, including tyrosine phosphorylation of transcription factor STAT5 and mitogen-activated protein kinase (MAPK) Erk1/2 activity as well as bcl-2 family member bcl-xL.
Materials and Methods
Reagents
Matrine was provided by the Ningxia Yanchi Pharmaceutical Company (Yanchi, China) and prepared as a 10 mg/ml stock solution in sterile H2O. Imatinib was purchased from Novartis Pharmaceuticals (Basel, Switzerland) and prepared as a 5 mM stock solution in sterile H2O.
Cell culture
K562 cells were cultured in RPMI 1640 supplemented with penicillin, streptomycin, and 10% FCS. They were maintained in a 37°C, 5% CO2, fully humidified incubator, passed twice weekly. Logarithmically growing cells were collected at a concentration of 1 × 105 cells/ml,to which were added the designated drugs, and the cells were placed back into the incubator for another 48 h. At the end of the incubation period, cells were transferred to sterile centrifuge tubes, pelleted by centrifugation, and prepared for analyses described below.
Annexin V-FITC/propidium iodine Fluorescence Activated Cell Sorter (FACS)
Apoptosis of each sample was determined by flow cytometry using a commercially available (Bender MedSystems Inc., Vienna, Austria) Annexin V-FITC/propidium iodine apoptosis detection kit. After drug treatment, cells were washed twice in ice-cold PBS and resuspended in 250 µl of binding buffer at 1 × 105 cells/ml. A 100-µl suspension was taken and incubated with 5 µl Annexin V/FITC and 10 µl propidium iodine (20 µg/ml) in the dark for 15 min at room temperature. Finally, 400 µl of PBS was added to each sample, and samples were analyzed by flow cytometry and evaluated based on the percentage of cells staining for Annexin V (apoptotic cells).
Western blot analyses
A modified method as previously described was used (Dorsey et al., Citation2000). Briefly, collected cells were lysed immediately in buffer [1% Triton X-100, 150 mM NaCl, 25 mM Tris-HCl (pH 7.2), 0.5 mM EDTA, 0.5 mM Na3VO4] supplemented with a protease inhibitor cocktail (Roche Molecular Biochemicals, Manheim, Germany). Protein concentration was determined (Micro BCA kit; Beyotime Biotechnology, Nanjing, China). Equal amounts of protein (60 µg) were boiled for 5 min, separated by SDS-PAGE, and electroblotted to nitrocellulose membrane. After blocking, the blots were incubated with an appropriate dilution of specific antisera or monoclonal antibodies (PARP, bcl-xL, phospho-Erk1/2, phospho-STAT5, Cell Signaling Technology, Danvers, USA; bcr/abl P210, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 1 h at room temperature. Blots were washed three-times and then incubated with a 1:2000 dilution of horseradish peroxidase–conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at room temperature. Blots were again washed three-times and then developed using a chemiluminescence assay. Blots for phospho-Erk1/2, phospho-STAT5, or other antibodies were stripped and reprobed for total-Erk 1/2, STAT-5, or β-actin (Cell Signaling Technology, Danvers, USA) to be used as a loading control.
Cytotoxicity assay
Cells in 96-well dishes were exposed to imatinib or/and matrine for 72 h. Cell viabilities were assessed by the 3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyl tetrazolium (MTT) assay (Mosmann, Citation1983). Briefly, K562 cells were collected and resuspended in 1640 medium at 1 × 105 cells/ml. A 90-µl aliquots was added to each well of 96-well plate, followed by addition of a 10-µl of ddH2O containing concentrations of drugs. Three replicate wells were used in each data point in the experiments. After incubation for 72 h, 10 µl of MTT solution (5 mg/ml in ddH2O) was added to each well, and plates were then incubated for 4 h at 37°C. Intracellular formazan crystals were dissolved by addition of 100 µl isopropanol with 0.04 N HCl to each well, until the solution turned purple and absorbance analyzed in an enzyme-linked immunosorbent assay (ELISA) plate reader at 570 nm.
Results
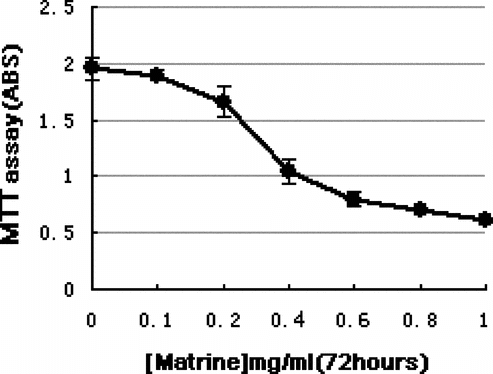
Matrine inhibits cell growth
The growth inhibition of K562 cells by matrine at various concentrations was determined by MTT. As shown in , matrine inhibited cell growth in a dose-dependent manner. Treatment of K562 cells with matrine at concentrations higher than 0.2 mg/ml for 72 h resulted in significant growth inhibition. The IC50 concentration (defined as the concentration of the drug at which 50% inhibition is observed, that is, [mean OD (untreated)-mean OD (drug-treated)]/mean OD (untreated) × 100 = 50%) for the growth inhibition of K562 cells by matrine is 0.44 mg/ml (calculated from the plotted data).
Matrine enhances growth inhibition and apoptosis induction by imatinib
Recent reports have indicated that some agents promote growth inhibition and apoptosis induction by imatinib in leukemia cells. In this study, we determined whether matrine would have similar effects on K562 cells. MTT assay demonstrated that the 0.2 mg/ml matrine was required to enhance imatinib-induced growth inhibiton in K562 cells (data not shown). As shown in A, simultaneous exposure to both matrine (0.2 mg/ml) and imatinib at low dose (0.2 µM) for 48 h resulted in promotion of growth inhibition. The results from FACS also showed apoptotic populations increased to 26.8% after such a combined treatment. However, separated treatments of K562 cells with either 0.2 mg/ml matrine or 0.2 µM imatinib for 48 h did not exhibit significant increase of apoptotic populations (B). Increased cleavage of PARP [poly (ADP-ribose) polymerase] into 85-kDa fragments was also observed in the combined treatment compared with each agent alone ().
Figure 2 Combined effects of matrine and imatinib on K562 cells (A) MTT assay showed the combined effects of 0.2 mg/mlmatrine with indicated concentrations of imatinib for 48 h on cell growth. (B) FACS demonstrated that the combination of treatment for 48 h increased apoptotic populations. The values represent means and standard deviations obtained from triple cultures
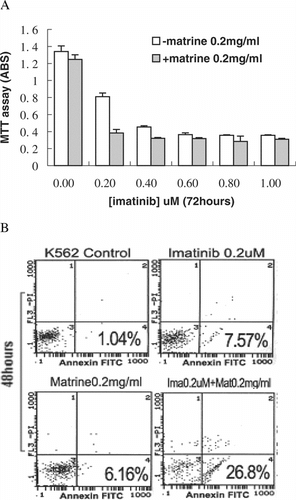
Figure 3 Western blots of several protein levels of bcr/abl mediating signal transduction pathways. Combined treatment increased PARP cleavage and downregulated protein levels of bcr/abl and phospho-Erk1/2 but did not alter those of phospho-STAT5, bcl-xL, total-Erk, and total-STAT5. Each experiment was repeated two times, and similar results were obtained
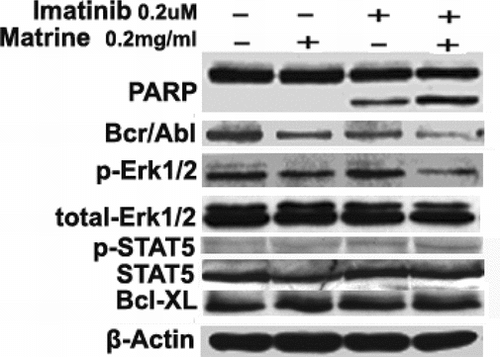
Matrine combined with imatinib downregulates protein levels of bcr/abl and phospho-Erk1/2
Effects of the combined treatment on several key proteins of bcr/abl mediating signal transduction pathways were then examined. As shown in , the downregulation of either bcr/abl or phospho-Erk1/2 by either imatinib or matrine alone at low dose was not noted, but a combined treatment downregulated both. However, the combined treatment did not change protein levels of bcl-xL and tyrosine phosphorylation of transcription factor STAT5.
Discussion
Our results show that K562 cells exposed to either imatinib or matrine for 48 h at lower concentration exhibit neither clear apoptotic populations nor alteration of key protein levels. However, cells treated by a combination of treatment showed significant apoptosis accompanied by a decrease of protein levels of bcr/abl and phospho-Erk1/2. Unexpectedly, the combination of treatment did not alter protein levels of bcl-xL and phosphoration of transcription factor STAT5.
Extracellular signal-related kinases (Erk1/2; P42/44 MAPK), one of three major subfamilies of the mitogen-activated protein kinases (MAPK), mediate signal pathways involved in cell survival and death. Although the modulation of Erk1/2 activity has been reported to be associated with antiapoptotic actions (Xia et al., Citation1995), the relationship between bcr/abl expression and Erk1/2 activity is still controversial. Morgan et al. found that there is no clear relationship between the expression of bcr/abl and the Erk1/2 activity in a panel of human leukemia cell lines (Morgan et al., Citation2001). However, Wang et al. reported that Erk1/2 is the downstream target of bcr/abl kinase (Wang et al., Citation1998), and Woessmann et al. found that the destruction of Erk1/2 through transfection with dominant-negative Erk1 mutant or treatment with inhibitor of Mek1/2, which is directly upstream of Erk1/2, induced apoptosis in K562 cells (Woessmann et al., Citation2001). In this study, we found that both bcr/abl protein level and the Erk1/2 activity were reduced in K562 cells exposed to the combination treatment of matrine and imatinib. The data provided the evidence that the modulation of Erk1/2 activity by apoptosis induction in this combination to K562 cells may be through bcr/abl-dependent pathways.
The bcl-2 family members including both antiapoptotic and proapoptotic proteins are key regulators of apoptosis, and the balance between these proteins is essential to cell fate (Adams et al., Citation1998). Apoptosis induction in K562 cells is usually associated with modulations on bcl-2 family members. Downstream signaling pathways mediated by the deregulated bcr/abl kinase activity in K562 cells lead to overexpression of the antiapoptotic bcl-xL but not bcl-2 (Ray et al., Citation1996; Amarante-Mendes et al., Citation1998). Our results show that the apoptosis induction by the combined treatment downregulated bcr/abl protein but did not reduce the protein level of bcl-xL. In addition, the unaffected bcl-xL level was consonant with the unaltered STAT5 activity, as the promoter of bcl-xL is recognized by the transcription factor STAT5 in K562 cells (Gesbert et al., Citation2000). Similar phenomenon was observed in arsenic trioxide–induced apoptosis in K562 cells, but the mechanism underlying its effect on bcr/abl versus. STAT5 remains unclear (Nimmanapalli et al., Citation2003). Our data indicate that matrine may have interacted with the signals mediated by bcr/abl to cause no alteration in the tyrosine phosphorylation of STAT5. This kind of action of combination may not be related to that of arsenic trioxide. Thus, further studies such as exploring the target protein of matrine in K562 cells are needed to elucidate the underlying mechanism of the effects.
Acknowledgments
We thank Prof. Ruolan Qian (Institute of Biochemistry and Cell Biology, Shanghai, P.R. China) for providing us with K562. This work was supported by grants from the National Natural Science Foundation of China, No. 30472185, from the Natural Science Foundation of Guangdong Province, P.R. China, No. 04106123, and from SRF for ROCS, SATCM.
References
- Adams JM, Cory S (1998): The Bcl-2 protein family: Arbiters of cell survival. Science 281: 1322–1326. [INFOTRIEVE], [CSA]
- Amarante-Mendes GP, Naekyung Kim C, Liu L, Huang Y, Perkins CL, Green DR, Bhalla K (1998): Bcr-Abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome C and activation of caspase-3. Blood 91: 1700–1705. [INFOTRIEVE], [CSA]
- Cai X, Wang GJ, Zai Y, et al. (1997): Analysis of efficacy of matrine injection in treatment of chronic hepatitis B. Acad J Second Military Med Univ 18: 47–49. [CSA]
- Daley G, Ven Etten R, Baltimore D (1990): Induction of chronic myelogenous leukemia in mice by the p210bcr/abl gene of the Philadelphia chromosome. Science 247: 824–830. [INFOTRIEVE], [CSA]
- Dhut S, Champlin T, Young BD (1990): BCR/ABL and ABL proteins: Biochemical characteization and localization. Leukemia 4: 745–750. [INFOTRIEVE], [CSA]
- Dorsey JF, Jove R, Kraker AJ, Wu J (2000): The pyrido[2,3-d.]pyrimidine derivative PD180970 inhibits p210Bcr-Abl tyrosine kinase and induces apoptosis of K562 leukemic cells. Cancer Res 60: 3127–3131. [INFOTRIEVE], [CSA]
- Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB (1996): Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nature Med 2: 561–566. [INFOTRIEVE], [CROSSREF], [CSA]
- Gesbert F, Griffin JD (2000): Bcr/Abl activates transcription of the Bcl-X gene through STAT5. Blood 96: 2269–2276. [INFOTRIEVE], [CSA]
- Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, Sawyers CL (2001). Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 293: 876–880. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Kano Y, Akutsu M, Tsunoda S, Mano H, Sato Y, Honma Y, Furukawa Y (2001): In vitro cytotoxic effects of a tyrosine kinase inhibitor STI571 in combination with commonly used antileukemic agents. Blood 97: 1999–2007. [INFOTRIEVE], [CROSSREF], [CSA]
- Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, Niederwieser D, Resta D, Capdeville R, Zoellner U, Talpaz M, Druker B, Goldman J, O'Brien SG, Russell N, Fischer T, Ottmann O, Cony-Makhoul P, Facon T, Stone R, Miller C, Tallman M, Brown R, Schuster M, Loughran T, Gratwohl A, Mandelli F, Saglio G, Lazzarino M, Russo D, Baccarani M, Morra E (2002): Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med 346: 645–652. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Liu XS, Jiang J (2005): Alterations in tyrosine protein phosphorylation induced by matrine in leukemia cells. Pharm Biol 43: 624–629. [CSA]
- Lugo TG, Pendergast AM, Muller AJ, Witte ON (1990): Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science 247: 1079–1082. [INFOTRIEVE], [CSA]
- Morgan MA, Dolp O, Reuter CW (2001): Cell-cycle-dependent activation of mitogen-activated protein kinase kinase (MEK-1/2) in myeloid leukemia cell lines and induction of growth inhibition and apoptosis by inhibitors of RAS signaling. Blood 97: 1823–1834. [INFOTRIEVE], [CROSSREF], [CSA]
- Mosmann T (1983): Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Immunol Methods 65: 55–63. [CROSSREF], [CSA]
- Nimmanapalli R, Bali P, O'Bryan E, Fuino L, Guo F, Wu J, Houghton P, Bhalla K (2003): Arsenic trioxide inhibits translation of mRNA of bcr-abl, resulting in attenuation of Bcr-Abl levels and apoptosis of human leukemia cells. Cancer Res 63: 7950–7958. [INFOTRIEVE], [CSA]
- Niu KZ (1997): Pharmacology and clinical application of Sophora flavescents.. Int J Oriental Med 22: 75–81. [CSA]
- Nowell PC, Hungerford DA (1960): A minute chromosome in human chronic granulocytic leukemia. Science 132: 1497–1499. [CSA]
- Ray S, Bullock G, Nunez G, Tang C, Ibrado AM, Huang Y, Bhalla K (1996): Enforced expression of Bcl-xS induces differentiation and sensitizes CML-blast crisis K562 cells to Ara-C mediated differentiation and apoptosis. Cell Growth Differ 7: 1617–1623. [INFOTRIEVE], [CSA]
- Roumiantsev S, Shah NP, Gorre ME, Nicoll J, Brasher BB, Sawyers CL, Van Etten RA (2002): Clinical resistance to the kinase inhibitor STI-571 in chronic myeloid leukemia by mutation of Tyr-253 in the Abl kinase domain P-loop. Proc Natl Acad Sci USA 99: 10700–10705. [INFOTRIEVE], [CROSSREF], [CSA]
- Thiesing JT, Ohno-Jones S, Kolibaba KS, Druker BJ (2000): Efficacy of STI571, an abl tyrosine kinase inhibitor, in conjunction with other antileukemic agents against Bcr-Abl-positive cells. Blood 96: 3195–3199. [INFOTRIEVE], [CSA]
- Wang S, Guo CY, Castillo A, Dent P, Grant S (1998): Effect of bryostatin 1 on taxol-induced apoptosis and cytotoxicity in human leukemia cells (U937). Biochem Pharmacol 56: 635–644. [INFOTRIEVE], [CROSSREF], [CSA]
- Woessmann W, Miveschi NF (2001): Role of ERK activation in growth and erythroid differentiation of K562 cells. Exp Cell Res 264: 193–200. [INFOTRIEVE], [CROSSREF], [CSA]
- Xia Z, Dickens M, Raingeaud J, Davis RJ (1995): Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270: 1326–1331. [INFOTRIEVE], [CSA]
- Zhang LP, Jiang JK, Tam JW, Zhang Y, Liu XS, Xu XR, Liu BZ, He YJ (2001): Effects of matrine on proliferation and differentiation in K-562 cells. Leuk Res 25: 793–800. [INFOTRIEVE], [CROSSREF], [CSA]