Abstract
Three new lactone derivatives, (3E.)-5,6-dihydroxy-3-(3-hydroxy-4-nonyl-5-oxofuran-2(5H.)-ylidene)-7-nonyl-1-benzofuran-2(3H.)-one (1), (3E.)-5,6-dihydroxy-3-(3-hydroxy-4-decyl-5-oxofuran-2(5H.)-ylidene)-7-decyl-1-benzofuran-2(3H.)-one (2), and (3E.)-5,6-dihydroxy-3-(3-hydroxy-4-undecyl-5-oxofuran-2(5H.)-ylidene)-7-undecyl-1-benzofuran-2(3H.)-one (3), denominated as parathesilactones A, B, C, respectively, and two new quinone derivatives, (2,7,8-trihydroxy-3,6-didecyldibenzo[b,d.]furan-1,4-dione (4) and (2,7,8-trihydroxy-3,6-dinonyldibenzo[b,d.]furan-1,4-dione) (5), denominated as parathesiquinones A, B, respectively, were isolated from the branches of Parathesis amplifolia. Lund. (Myrsinaceae). In addition, three known triterpenes, α.-amyrin (6), β.-amyrin (7), and bauerenol (8), and four known alkenylresorcinols, 5-pentadec-8-enyl resorcinol (9), 5-pentadec-10-enyl resorcinol (10), 5-heptadec-8-enyl resorcinol (11), and 5-heptadec-10-enyl resorcinol (12), were also isolated. The structure elucidation was achieved by means of MS, GC-MS, IR, 1H, 13C spectroscopy including Heteronuclear Multiple Quantum Coherence (HMQC), and Heteronuclear Multiple Bond Correlation (HMBC) techniques.
Introduction
Parathesis amplifolia. Lund. (Myrsinaceae) is an endemic tree, 15–20 ft high, with brown inflorescence and brown leaf underneath (Lundell, Citation1971; Ricketson, Citation1997). In our ongoing study of Panamaniam flora, the chloroform extract of the branches of Parathesis amplifolia. resulted in the isolation of three novel parathesilactones A–C: [(3E.)-5,6-dihydroxy-3-(3-hydroxy-4-nonyl-5-oxofuran-2(5H.)-ylidene)-7-nonyl-1-benzofuran-2(3H.)-one (1), (3E.)-5,6-dihydroxy-3-(3-hydroxy-4-decyl-5-oxofuran-2(5H.)-ylidene)-7-decyl-1-benzofuran-2(3H.)-one (2), and (3E.)-5,6-dihydroxy-3-(3-hydroxy-4-undecyl-5-oxofuran-2(5H.)-ylidene)-7-undecyl-1-benzofuran-2(3H.)-one (3)], and two new parathesiquinones A, B [(2,7,8-trihydroxy-3,6-didecyldibenzo[b,d.]furan-1,4-dione (4) and (2,7,8-trihydroxy-3,6-dinonyldibenzo[b,d.]furan-1,4-dione) (5)] (), in addition to three known triterpenes (6–8) and four alkenylresorcinols (9–12).
Materials and Methods
General experimental procedures
IR spectra were recorded in KBr pellets using a Jasco (Japan) FT/IR-230 spectrometer. NMR spectra were measured on a Varian (USA) UNITY 500 spectrometer in CDCl3 or DMSO at 500 MHz and 125 MHz for 13C as solvent with TMS as internal standard. Mass spectra were obtained on a Jeol (Japan) JMX-AX 505 HAD at 70 eV. GC-MS measurements were performed on a Shimadzu (Japan) 17-A gas chromatograph attached to a Jeol Automass System II, equipped with a capillary column (30 m × 0.25 mm narrow bore), film 0.25 mm (J&W Scientific (USA)); elution was carried out at the temperatures specified and using He as carrier gas at 50 cm/s. For the analysis of the alkenylresorcinols, the temperature was set from 200 to 290°C, at 3 degrees per min, total time 30 min; and for the analysis of the triterpene mixture, starting from 150 to 300°C, at 10 degrees per min, then 15 min at 300°C. Silica gel, glass-backed plates (Merck, Kieselgel 60 F254 or RP18 F254 S, 0.25-mm thickness) were used for thin-layer chromatography (TLC), and the compounds were detected by means (254 nm and 365 nm) of ultraviolet light and were sprayed with anisaldehyde–5% H2SO4). Silica gel 60 [Fuji Silycia (70–230 mesh)] was used for column chromatography (CC, Chemglass, USA). Low-pressure liquid chromatography was carried out on Lobar columns LiChroprep RP-8 (Merck, size A).
Plant material
Parathesis amplifolia. Lund. was collected in Altos de Campana National Park, Myrsinaceae Trail, on July 18, 1996, a cloud forest 1000 m above sea level, in the Province of Panama. The taxonomic identification was established by Prof. Mireya D. Correa, Director of the Herbarium of the University of Panama (PMA), where voucher specimens (FLORPAN 2636) are deposited.
Extraction and isolation
The dried powdered branches (234 g) of P. amplifolia. were extracted with CHCl3 by percolation at room temperature, and the extract was filtered and concentrated to obtain a residue (6.92 g, 2.95%). The chloroform extract was subjected to fractionation by column chromatography, using silica gel (150 g) and benzene-acetone (100 to 80:20) as eluent to give five major fractions. Repeated open column chromatography of fraction 2 yielded 35.6 mg of compound 1 (fr. 2a) and 25 mg of a triterpene mixture (6–8) (fr. 2b). From fraction 3, after repeated Lobar RP-8 column (Merck) using MeOH-H2O (9:1) as eluent, compound 4 was obtained. By repeated Lobar RP-8 column (Merck) of the fraction 5 using MeOH-H2O (9:1) as eluent, two oily fractions (fr. 5a) and (fr. 5b) were obtained. The GC-MS (EI-MS) analysis of the dimethyldisalfide derivatives (DMDS) derivatives of the alkenylresorcinols series was carried out for resolving the mixture and determining the homologous long chains and the position of the double bond in compounds 9, 10 (fr. 5a) and compounds 11, 12 (fr. 5b), respectively.
Preparation of methyl derivatives
Ten milligrams of parathesiquinone B (5) were dissolved in 2.0 ml of MeOH, and 1 ml of trimethylsilyldiazomethane (10% in n.-hexane) (TCI) was added and stirred overnight. The solvent was evaporated and the derivative purified either by small column chromatography or pTLC.
Preparation of benzoyl derivatives of triterpenoids
Thirty milligrams of a mixture of three triterpenes were dissolved in 0.5 ml of pyridine, and 0.5 ml of benzoyl chloride (Nacalai Tesque, Kyoto, Japan) were added on an ice bath. After the mixture was allowed to stand at room temperature for 20 h, it was poured on ice-cold water and 20 ml of diethyl ether was added. The aqueous layer was further washed with diethyl ether twice consecutively. The organic fraction was washed with NaHCO3 1% (3 × 20 ml) and concentrated in vacuo. and chromatographed on pTLC (0.5 µ, 20 × 20 cm, silica gel 60 F254, Merck) using benzene as eluent. The upper layer yielded 6.1 mg and showed to be a mixture of α.-and β.-benzoyl amyrin, the lower (21 mg) was the third compound unknown as yet. To 10.0 mg of the latter, 5 ml of KOH 1 N in MeOH was added and heated at 70°C for 2 h. The solvent was removed and the residue partitioned between water and benzene; the organic layer was dried to afford 7.03 mg of the bauerenol.
Preparation of DMDS derivatives for alkenylresorcinols
One milligram of each compound was dissolved in 100 µl of hexane and 100 µl of DMDS (dimethyl disulfide) (Nacalai Tesque, Kyoto, Japan) and 20 µl of iodine solution (60 mg of I2 in 1 ml of diethyl ether). The reaction was carried out in a 2-ml glass tube tightly closed with a Teflon cap and kept for 18 to 20 h at 50°C. Samples were diluted with 200 µl of Na2S2O3 solution (5% in distilled water). The organic phase was removed with a Pasteur pipette and the aqueous layer extracted again with n.-hexane. The sample was applied to a small pTLC plate (5 × 5 cm) and developed using benzene-acetone (98:2) as solvent. The main band was recovered and extracted with the same solvent. The EIMS of the residue was obtained to determine the position of the double bond.
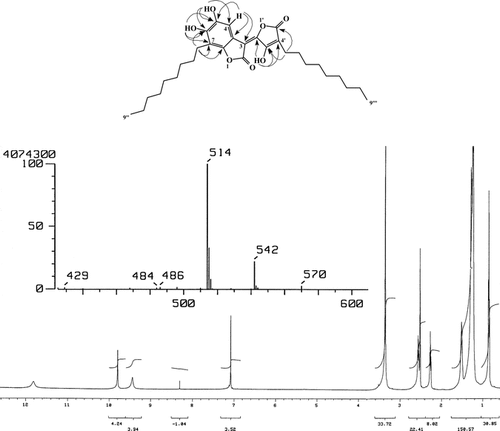
Parathesilactones (A–C) (1, 2, 3) (3E.)-5,6-dihydroxy-3-(3-hydroxy-4-nonyl-5-oxofuran-2(5H.)-ylidene)-7-nonyl-1-benzofuran-2(3H.)-one (1), (3E.)-5,6-dihydroxy-3-(3-hydroxy-4-decyl-5-oxofuran-2(5H.)-ylidene)-7-decyl-1-benzofuran-2(3H.)-one (2), (3E.)-5,6-dihydroxy-3-(3-hydroxy-4-undecyl-5-oxofuran-2(5H.)-ylidene)-7-undecyl-1-benzofuran-2(3H.)-one (3): Red deliquescent needles from CHCl3 35.6 mg, Ir ν (KBr) cm−1: 3520, 3320, 2840, 2920, 2960, 1760, 1720, 1650, 1625, 1495, 1320, 1020. HRMS: m/z. calculated 514.2930 (C30H42O7) found 514.2906; m/z. calculated 570.3556 (C34H50O7) found 570.3507; m/z. calculated 542.3244 (C32H46O7) found 542.3289 EIMS (70 eV): m/z. (rel. int. %) 570 (5), 542 (20), 514 (100), 401 (12), 318 (30), 292 (10), 289 (10), 205 (12), 195 (7). 1H NMR (500 MHz, DMSO): δ. 11.81 (1H, s, OH-3′), 9.79 (1H, s, OH-5), 9.44 (1H, s, OH-6), 7.07 (1H, s, H-4), 2.55 (2H, t, J. = 7.5 Hz, CH2-1″), 2.26 (2H, t, J. = 7.4, CH2-1‴), 1.51 (4H, m, CH2-2′H, 2‴), 1.28–1.24 (24H, br s, 12(CH2), 0.838 (6H, t, J. = 6.6, 2CH3). 13C NMR (125.75, MHz, DMSO): δ. 172.97 (C-2), 167.43 (C-5′), 161.76 (C-3′), 148.16 (C-2′), 147.82 (C-6), 146.86 (C-7a), 142.92 (C-5), 113.56 (C-7), 110.81a (C-3a), 107.62a (C-3), 107.61 (CH-4), 106.37 (C-4′), 23.32 (CH2-1″), 22.19 (CH2-1‴), 14.03 (CH3-9″, 9‴), 31.36, 29.05, 28.98, 28.93, 28.80, 28.77, 28.68, 28.41, 26.96, 21.44, 21.30 signals for the side chain as CH2. Carbon signal may be interchangeable. HMQC and HMBC were used to confirm these assignments.
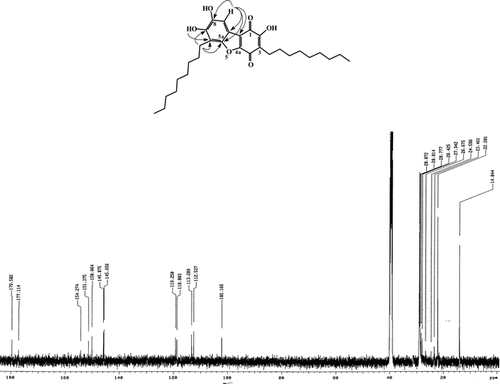
Parathesiquinone A (2,7,8-trihydroxy-3,6-didecyldibenzo[b,d.]furan-1,4-dione) (4): 10 mg, dark red gum. HRMS: m/z. calculated 498.2981 (C30H42O6) found 498.2594. EIMS (70 eV): m/z. (rel. int. %) 498 (100), 442 (30), 441 (80), 386 (35), 300 (20), 273 (75), 232 (60), 135 (40), 91 (100). 1H NMR (500 MHz, CDCl3): δ. 7.3 (1H, s, H-9), 2.90 (2H, br s, H-1″), 2.52 (2H, br s, H-1‴), 1.25 (24H, br s, side chain), 0.87 (6H, t, CH3). 1H NMR (500 MHz, D6MSO): δ. 10.79 (1H, s, OH-C-2), 10.03 (1H, s, OH C-7), 9.07 (1H, s, OH-C-8), 7.17 (1H, s, H-9), 2.78 (2H, t, H-1″), 2.50 (4H, br s, H-2″, H-2‴), 2.35 (2H, t, H-1‴), 1.28–1.19 (24H, br s, side chain), 0.826 (6H, t, CH3). 13C NMR (125.75, MHz, D6MSO): δ. 179.58 (C-1), 177.11 (C-3), 154.27 (C-2), 151.37 (C-4a), 150.06 (C-5a), 145.87 (C-8), 145.66 (C-7), 119.25a (C-1a), 118.80 (C-3), 113.29a (C-9a), 112.53 (C-6), 102.17 (C-9), 32.16, 29.94, 29.91, 29.85, 29.74, 29.624, 29.41, 28.698, 22.96, 14.42 (side chains).
Parathesiquinone B (2,7,8-trihydroxy-3,6-dinonyldibenzo[b,d.]furan-1,4-dione) (5): 3 mg, dark red gum. HRMS: m/z. calculated 526.3294 (C32H46O6) found 526.3211. EIMS (70 eV): m/z. (rel. int. %) 526 (100), 414 (30), 386 (25), 273 (15). NMR (500 MHz, CDCl3): δ. 7.28 (1H, s, H-9), 2.90 (2H, br s, H-1″), 2.52 (2H, br s, H-1‴), 1.25 (28H, br s, side chain), 0.87 (6H, t, CH3). Carbon signal may be interchangeable. HMBC was used to confirm these assignments.
Methylparathesiquinone B (5a): Orange gum. NMR (500 MHz, CDCl3): δ.7.36 (1H, s), 4.10 (3H, s), 3.96 (3H,s), 3.89 (3H, s), 2.92 (2H, t, J. = 7.8), 2.52 (2H, t, J. = 7.7), 1.678–1.647(4H, m), 1.25 (24H, br s), 0.876 (6H, m).
Triterpene mixture (6, 7, 8): α., and β.-Amyrin (6, 7) and bauerenol (8) mixture. 25 mg, colorless needles from CHCl3/MeOH. Ir υ(KBr) cm−1: 3400, 2950, 1460, 1380, 1030. EIMS (70 eV): m/z. (rel. int. %) 426 (50), 411 (25), 247 (60), 218 (100). 1H NMR (500 MHz, CDCl3): δ. 5.43–5.407 (1H, m, H-7 bauerenol), 5.19–5.18 (1H, m, H-11β.-amyrin), 5.13–5.12 (1H, m, H-11α.-amyrin).
Bauerenol (8): 7.03 mg, white solid. [α.]D (CHCl3, C = 3.51 mg/ml) − 20.2. EIMS (70 eV): m/z. (rel. int. %) 426 (60), 247 (90), 229 (50), 218 (30), 205 (25). 1H NMR (500 MHz, CDCl3): δ. 5.42 (1H, dd, J. = 2.8, 6.8, H-7), 3.27–3.23 (1H, m, H-3), 1.03, 0.99, 0.97, 0.94, 0.86, 0.74 (3H each, s, CH3), 1.05, (3H, d, J. = 6.8, CH3), 0.95 (3H, d, J. = 5.8, CH3). 13C NMR (125, CDCl3): δ. 145.34, 116.42, 79.25, 54.86, 50.39, 48.20, 41.22, 38.87, 37.99, 37.71, 37.67, 36.86, 35.31, 35.19, 32.39, 32.03, 31.50, 29.21, 28.85, 27.67, 27.53, 25.63, 24.14, 23.65, 22.65, 22.56, 16.79, 14.66, 12.98.
Benzoylbauerenol. (8a): 21 mg, white solid. EIMS (70 eV): m/z. (rel. int. %) 530 (75), 351 (100), 229 (70), 105 (100). 1H NMR (500 MHz, CDCl3): δ. 8.05 (2H, dd, J. = 0.86, 7.48, H-2′), 7.55 (1H, t, J. = 7.7, H-4′), 7.45 (2H, t, J. = 7.7, H-3′), 5.44–5.43 (1H, m, H-7), 4.77 (1H, dd, J. = 4.4,11.6, H-3), 1.09, 1.04, 1.012, 0.97, 0.93, 0.82 (6H/each, s, CH3), 1.05 (3H, d, J. = 7.0, CH3), 0.91 (3H, d, J. = 5.7, CH3).
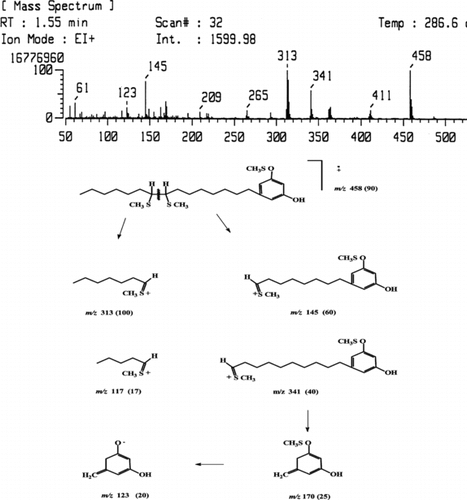
5-Pentadecenylresorcinol (9, 10): 30.0 mg, colorless oil. EIMS (70 eV): m/z. (rel. int. %) 318 (90), 222 (38), 205 (15), 191 (23), 163 (23), 137 (50), 125 (40), 124 (100), 123 (70). 1H NMR (500 MHz, CDCl3): δ. 6.23 (2H, s, H-4,6), 6.17 (1H, s, H-2), 5.36–5.33 (2H, m, H-8′,9′), 2.47 (2H, t, J. = 7.7, H-1′), 2.02–1.99 (4H, m, H-7′, 10′) 1.57–1.53 (2H, m, H-2′), 1.29 (16H, br s, H-on side chain), 0.88 (3H, t, J. = 7.1, 3.8, H-15 =). 13C NMR (125.75, MHz, CDCl3): δ. 156.58 (C-1), 100.28 (C-2), 156.58 (C-3), 108.14 (C-4), 146.17 (C-5), 108.14 (C-6), 36.09 (C-1′), 27.47 (C-7′), 129.92 (C-8′), 130.06 (C-9′), 27.50 (C-10′), 14.42 (Me), 32.05, 31.32, 30.03, 29.67, 29.58, 29.53, 29.50, 29.28, 22.95.
DMDS-5-Pentadecenylresorcinol (9, 10) EIMS (70 eV): m/z. (rel. int. %) 458 (90), 341 (40), 313 (100), 170 (25), 145 (60), 117 (20), 123 (20).
5-Heptadecenylresorcinol (11, 12): 12.0 mg, colorless oil. EIMS (70 eV): m/z. (rel. int. %) 346 (100), 250 (30), 205 (15), 166 (23), 163 (20), 137 (50), 125 (25), 124 (100), 123 (70). 1H NMR (500 MHz, CDCl3): δ. 6.23 (2H, s, H-4,6), 6.17 (1H, s, H-2), 5.36–5.33 (2H, m, H-8′,9′), 2.47(2H, t, J. = 7.7, H-1′), 2.02–1.98 (4H, m, H-7′, 10′) 1.56–1.51 (2H, m, H-2′), 1.29 (20H, br s, H-on side chain), 0.88 (3H, t, J. = 7.1, 6.0, H-15′). 13C NMR (125.75, MHz, CDCl3): δ. 156.58 (C-1), 100.28 (C-2), 156.58 (C-3), 108.14 (C-4), 146.17 (C-5), 108.14 (C-6), 36.09 (C-1′), 27.47 (C-9′), 129.92 (C-10′), 130.06 (C-11′), 27.50 (C-12′), 14.42 (Me), 32.05, 31.32, 30.03, 29.67, 29.58, 29.53, 29.50, 29.28, 22.95.
DMDS-5-Heptadecenylresorcinol (11, 12): EIMS (70 eV): m/z. (rel. int. %) 486 (60), 341 (60), 313 (100), 173 (35), 170 (50), 145 (35), 123 (20).
Results and Discussion
A mixture of three novel lactone derivatives (1–3) and a mixture of two novel quinone derivatives (4, 5); a mixture of three triterpenes α. and β.-amyrin (6, 7), bauerenol (8), and a mixture of four alkenylresorcinols [5-pentadec-8-enyl resorcinol (9), 5-pentadec-10-enyl resorcinol (10), 5-heptadec-8-enyl resorcinol (11), and 5-heptadec-8-enyl resorcinol (12)] were isolated and characterized as described earlier.
The IR spectrum of compound 1 showed absorption bands typical of C = O 1760, 1720 cm−1 of five-membered lactone ring. The HR mass spectrum showed a [M]+ peak at m/z. 514.2906 corresponding with the molecular formula C30H42O7. The 1H NMR spectrum (DMSO, 500 MHz) showed signals for three OH groups at δ. 11.81, 9.79, and 9.44. A one-proton signal in the aromatic region (δ. 7.07) was assigned to the C-4 proton, while the triplets integrating for 2 protons at δ. 2.55 and 2.26 were assigned to the protons on the C-1″ and C-1‴ in the side chains. A four proton multiplet at δ. 1.51 (4H) was assigned to the C-2″ and C-2‴ protons, the broad singlet (δ. 1.28–1.24) was assigned to the protons on the side chain, and, a triplet integrating for 6 protons at δ. 0.838 was assigned to the methyl groups at the end of the side chains. 13C NMR spectrum showed 12 carbons in the aromatic region, of which 11 were quaternary, based and HMQC and HMBC correlations, a CH signal at δ. 107.61 was assigned to C-4. The signals at δ. 172.97 and 167.76 were assigned to carbonyl group C-2 and C-5′, respectively. While signals at 161.76, 148.16, 147.82, 146.86, and 142.92 corresponded with aromatic carbons bearing oxygens, there were signals for the CH2 and methyl groups of the side chain.
Comparison of the 13C NMR of 1 with that of embeline reported by Kiuchi et al. (Citation1998aCitation1998b) and the resorcinols series with the same nucleus but without the side chains reported by Cirigottis et al. (Citation1974), together with the HMBC correlations allowed us to establish the structure as parathesilactone A (3E.)-5,6-dihydroxy-3-(3-hydroxy-4-nonyl-5-oxofuran-2(5H.)-ylidene)-7-nonyl-1-benzofuran-2(3H.)-one) (1), unequivocally (). In addition to the peak at [M]+ 514.2906, the EIMS showed two peaks at 542.3289 and 570.3507 corresponding with the formula C32H46O7 and C34H50O7, respectively. This data suggests that 1 is a mixture of three homologous compounds differing in the length of the side chain, because cleavage of two CH2 is not observed in long side chains. Therefore, parathesilactone B (3E.)-5,6-dihydroxy-3-(3-hydroxy-4-decyl-5-oxofuran-2(5H.)-ylidene)-7-decyl-1-benzofuran-2(3H.)-one) (2) and parathesilactone C (3E.)-5,6-dihydroxy-3-(3-hydroxy-4-undecyl-5-oxofuran-2(5H.)-ylidene)-7-undecyl-1-benzofuran-2(3H.)-one) (3) have two side chains of 10 and 11 carbons, respectively.
The HR mass spectra of 4 showed a [M]+ peak at m/z 526.3211 corresponding with the formula C32H46O6. 1H NMRshowed a broad signal at δ.10.79 assigned to the OH on C-2 and two singlets at δ.10.03 and 9.07 were assigned to OH bound to C-7 and C-8, respectively. The singlet at δ. 7.17 for an isolated aromatic proton was assigned to proton on C-9, which showed correlation with the signal at δ.102.17 (C-9) In the HMQC spectrum, two triplets, integrating for two protons, at δ. 2.78 and 2.35 were assigned to the protons on C-1″ and C-1‴ in the side chain. A broad singlet, integrating for 4 protons (δ. 2.50), was assigned to the protons on the second carbon in each of the two side chains. There were signals for the protons in the side chains (δ. 1.28–1.19) and for the methyl terminal of the side chains as triplet at δ. 0.826. 13C NMR showed 12 carbon signals in the aromatic region, 11 being quaternary carbons. The signals at δ. 179.58 and 177.11, corresponding with a quinone system, were assigned to C-1 and C-4, respectively. Signals occurred at 154.27 (C-2), 151.37 (C-4a), 150.06 (C-5a), 145.87 (C-8), 145.66 (C-7), indicating aromatic carbons bearing oxygen. There were signals for the CH2 and methyl groups of the side chain. The 13C NMR was compared with a synthetic compound with the same nucleus but without side chains together with its own HMBC correlations. Comparison of the13C NMR of 4 with the resorcinols series reported for Cirigottis et al. (Citation1974) and by comparison with embeline reported by Kiuchi et al. (Citation1998aCitation1998b) established the structure of parathesiquinone A (2,7,8-trihydroxy-3,6-didecyldibenzo[b,d.]furan-1,4-dione) (4), unequivocally (). In addition to the peak at [M]+ 526.3211, the EIMS showed a peak at m/z. 498.2594 corresponding with the formula C30H42O6. This suggests that 4 is a mixture of two homologous compounds differing in the length of the side chain, because cleavage of two CH2 is not observed in long side chains. Therefore, parathesiquinone B (2,7,8-trihydroxy-3,6-dinonyldibenzo[b,d.]furan-1,4-dione) (5) has two side chains of 9 carbons each.
It has been proposed by Kiuchi et al. (Citation1998aCitation1998b) that this series of lactones is formed during the extraction procedure, as demonstrated in the decoction of Embelia ribes., which contains embelin-type benzoquinones. Nevertheless, the parent compound in our sample was not detected. However, a homologue (13, 14, and 15 carbons in the side chain) mixture was found in high concentration in the extract from the stembark of P. amplifolia., obtained in the similar manner by percolating with chloroform at low temperature. Determinations of lactone were carried out by extracting, on ice, small amounts of freshly collected plant material and injecting into a HPLC-MS; the compound was detected in high quantities, establishing its natural occurrence.
Fraction 2a, which on TLC showed a single spot, turned out to be an unresolved mixture of three triterpenes. On GC-MS, it showed the presence of α. and β. amyrin, 6 and 7, by comparison with the authentic samples. However, the third compound could not be identified by this method. For this, the mixture was benzoylated, the components were isolated by pTLC, then hydrolyzed to obtain the parent compound. EIMS, 1H and 13C NMR data confirmed its structure as bauerenol 8, a very well-known triterpene (Meksuriyen et al., Citation1986).
Fractions 5a and 5b are very well-known 5-alkenylresorcinols, having a side chain of 15 and 17 carbons, respectively. Nevertheless, the fraction 5a is an isomeric mixture of 5-pentadec-8-enyl-resorcinol (9) and 5-pentadec-10-enyl-resorcinol (10) (Occolowitz & Wright, Citation1962; Cirigottis et al., Citation1974; Tyman & Colenut Citation1981), in accordance with the EIMS fragmentation pattern of the DMDS derivative. The fragmentation for the 5-pentadecenylresorcinols appears in . Also, fraction 5b proved to be a mixture of two isomeric known compounds, 5-heptadec-8-enyl-resorcinol (11) and 5-heptadec-10-enyl-resorcinol (12) (Cirigottis et al., Citation1974; Itokawa, et al., Citation1987).
Acknowledgments
We thank the International Foundation for Science for funding a project (grant no. #F/1081-2) (P.N.S) and the International Matsumae Foundation of Japan for the postdoctoral fellowship grant to P.N.S. Thanks are also due to Prof. Mireya D. Correa for the taxonomic identification of the plant, to Carlos Guerra and Alex Espinosa for their help in plant collection, and to the Organization of American States for supporting CIFLORPAN.
References
- Cirigottis KA, Cleaver L, Corrie JET, Grasby RG, Green GH, Mock J, Nimgirawath S, Read RW, Ritchie E (1974): Chemical studies of the Proteaceae. Vll. Examination of the woods of 17 species for resorcinol derivatives. Aust J Chem 27: 234–255. [CSA]
- Itokawa H, Tosuka N, Nakahara K, Takeya K, Lepoittevin JP, Asakawa Y (1987): Antitumour principles from Ginkgo biloba. L. Chem Pharm Bull 35: 3016–3020. [INFOTRIEVE], [CSA]
- Kiuchi F, Suzuki N, Fukumoto Y, Goto Y, Mitsui M, Tsuda Y (1998a): Chemical transformation of embelin through epimerization during preparation of a decoction. Chem Pharm Bull 46: 1225–1228. [CSA]
- Kiuchi F, Takashima H, Tsuda Y (1998b): Dimerization of 2,5-dihydroxybenzoquinones in water. Chem Pharm Bull 46: 1229–1234. [CSA]
- Lundell CL (1971): Family 150. Myrsinaceae. In: Woodson RF, Schery R, and Collaborators. Annals of Missouri Botanical Garden, Vol. 58. Flora of Panama. Part VIII, pp. 285–353.
- Meksuriyen D, Nanayakkara NPD, Phoebe CH, Cordell GA (1986): Two triterpenes from Davidsonia pruriens.. Phytochemistry 25: 1685–1689. [CSA], [CROSSREF]
- Occolowitz JL, Wright AS (1962): 5-(10-Pentadecenyl) resorcinol from Grevillea. pyramidalis.. Aust J Chem 15(4): 858–86l. [CSA]
- Ricketson JM, Pipoly, II, JJ (1997): Notes on neotropical Parathesis. (Myrsinaceae). Novon 7: 398–405. [CSA]
- Tyman JHP, Tychopoulos V, Colenutb BA (1981): Long-chain phenols. XXI. Quantitative analysis of the phenolic lipids in technical cashew nutshell liquid, from Anacardium occidentale. by HPLC. J Chromatogr 213: 287–300. [CSA], [CROSSREF]