Abstract
Chitosan nanoparticles loaded with lysozyme were prepared by means of an ionic gelation process of chitosan (CS) and tripolyphosphate (TPP) on mild condition. Observations by TEM showed that the spherical chitosan nanoparticles were 50–280 nm in diameter. This work focuses on the effects of molecular weight (Mv) of CS, content of CS, TPP, and initial lysozyme on encapsulation efficiency (EE), loading capacity (LC), release rate and activity of lysozyme in chitosan nanoparticles. Increasing Mv of chitosan, TPP content, or decreasing initial lysozyme content, EE was enhanced whereas when increasing chitosan content, EE first increased then decreased. LC increased as Mv of chitosan, content of TPP, or initial lysozyme increased or chitosan content decreased. The EE could be up to 47.3% and the LC could be 11.4%. The lysozyme release rate increased with decreasing the initial amount of lysozyme, Mv, or content of chitosan and increasing the amount of TPP. The relative activity of lysozyme in all the samples was maintained above 85%.
Keywords:
Introduction
Chitosan, a partially deacetylated derivative of chitin composed of N.-acetylglucosamine, has emerged as a significant biomaterials and pharmaceutical excipient for drug delivery because of its biocompatibility, biodegradability, low immunogenicity, and low cost. What is more, chitosan is a unique natural occurring cationic polysaccharide, which allows it to form water-insoluble ionic complexes with a variety of anionic substances in a very mild condition. For example, chitosan has been ionically crosslinked in the presence of sulfate, citrate, tripolyphosphate (Peng et al., Citation2005) and alginate (Haque et al., Citation2005) for medical applications. Nanoparticles are now a promising material for tissue engineering. Nanoparticles can not only alter and enhance the properties of polymer such as mechanical degradable properties as an important additive, but also protect and controll release of the bioactive substance as a delivery system (Williams, Citation2005). All these advantages are due to the high surface area to volume ratio of nanoparticles (Smith et al., Citation2003). Accordingly, bioactive drug-loaded chitosan nanoparticles are sure to be helpful to enhance certain properties of polymers, especially biodegradable polymers. In the current study, a kind of chitosan nanoparticle containing lysozyme is reported for the first time.
Materials and Methods
Materials
Chitosan (CS): degree of deacetylation (DD) of which was 95.7% with different Mv (1.6 × 104, 3.1 × 104, 5.5 × 104, 7.0 × 104). DD were determined by acid-base titration method (Wu, Citation2003), and Mv was obtained from viscosity measurements and the Mark-Houwink relationship (Wang, Citation1991). Lysozyme (20000 U/mg) was purchased from Sino-American Biotechnology Company, China. Micrococcus lysodeikticus. was purchased from Sigma, USA. All other chemicals were of reagent grade.
Preparation of lysozyme-loaded nanoparticles
Chitosan was dissolved in acetic aqueous solution at different concentrations containing different concentrations of lysozyme. Then, 2 ml of sodium tripolyphosphate (TPP) aqueous solution were added to 5 ml of chitosan solution with lysozyme under stirring at room temperature. Then an opalescent suspension was formed. A little aggregate in the solution was separated by sedimetration. The lysozyme-loaded nanoparticles were separated from the opalescent suspension medium by ultra centrifugation at 8500 × g and 4°C for 30 min. Then, the separated nanoparticles were lyophilized at − 60°C and kept at 4°C.
Morphology and structure characterization of nanoparticles
The morphology and size observations of the nanoparticles were performed by TEM (Philips TECNAI10, Netherlands). Chitosan nanoparticles separated from suspension were lyophilized, and their structure characterizations were taken with KBr pellets on FTIR (Bruker EQUINOX 55, Germany).
Evaluation of lysozyme loading capacity of the nanoparticles
Lyophilized nanoparticles (20 mg) were digested in 4 ml of 0.2 M HCl for about 6 h at 37°C under stirring until a clear solution was obtained. The amount of loaded lysozyme was measured at 570 nm by a microBCA protein assay (Pierce, Rockford, IL, USA). A calibration curve was made using the clear solution of nonloaded nanoparticles.
The lysozyme loading capacity (LC) and encapsulation efficiency (EE) of the nanoparticle were calculated from Eqs. (1) and (2) indicated below:
where W is the weight of loaded lysozyme; W0 is total weight of lysozyme; and Wn is weight of lysozyme-loaded nanoparticles
Evaluation of lysozyme release from the nanoparticles in vitro.
The lysozyme-loaded chitosan nanoparticles were placed into test tube with PBS and incubated at 37°C under stirring. At appropriate intervals, samples were centrifuged and certain volume of the supernatant was taken out and replaced by the same volume of fresh medium. The amount of lysozyme released from the nanoparticles was evaluated by the BCA protein assay. A calibration curve was made at each time interval using nonloaded nanoparticles as correction in order to correct the absorbency due to the chitosan.
Evaluation of relative activity (RA) of released lysozyme from the nanoparticles in vitro.
The activity of lysozyme was measured at 450 nm with a microplate reader (Thermo MULTI SKAN MK3, USA) by a Micrococcus lysodeikticus. assay (Lee & Yang, Citation2002). RA of each sample was calculated from the equation RA = (As/Af) × 100. Here, As refers to activity of sample and Af refers to activity of fresh lysozyme standards of which weight were the same as the samples.
Results and Discussion
It is well-known that lysozyme is a natural enzyme existing in the human body and can catalyze chitosan to hydrograde but can't affect chitosan with DD higher than 95% (Kurita et al., Citation2000; Xia & Su, Citation2002). As a result, the chitosan utilized in the experiment can't be degraded by the lysozyme it loaded.
Physicochemical characterizations of nanoparticles
Morphology of the nanoparticles
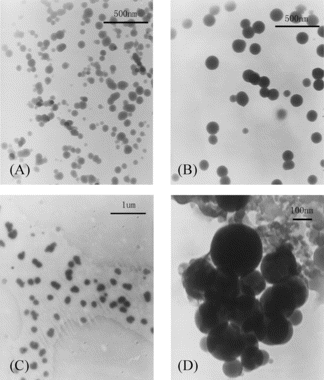
TEM images of the nanoparticles are shown in . The nanoparticles had a solid and consistent structure, and they were spherical in shape and about 50–280 nm in diameter. The Mv of CS, concentration of CS or TPP had significant influence on the size of the nanoparticles. As increasing the Mv of CS, concentration of CS or TPP, the size of nanoparticles increased (Calvo et al., Citation1997; Janes & Alonso, Citation2003).
FTIR spectra of the nanoparticles
The spectrum of pure chitosan exhibited an amine deformation peak at 1605 cm−1 and an amide I carbonyl stretch at 1643 cm−1 (Samuels, Citation1981). In the spectrum of the lysozyme-loaded chitosan nanoparticles, the fact that the peak 1227 cm−1 that represented the P = O group of TPP appeared (Mi, Citation1999), the amine deformation peak shifted from 1605 cm−1 to 1536 cm−1, and the peak at 1643 cm−1 developed into a sharp peak meant an interaction was occurring at the amine group on the chitosan, and this could represent the binding of tripolyphosphate to this site.
Encapsulation of lysozyme within nanoparticles
In order to investigate the effect of each factor on the encapsulation of lysozyme in nanoparticles, when one factor was tested, its own parameters were altered and kept other factors constant. Without specific notice, the parameters were as follows: Mv of chitosan was 5.5 × 104, concentration of chitosan was 1 mg/ml, concentration of TPP was 4 mg/ml, and initial concentration of lysozyme was 0.47 mg/ml.
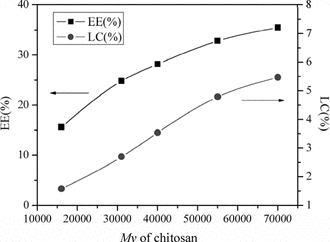
Effect of chitosan Mv in encapsulation of lysozyme
shows that when increasing the Mv of CS from 1.6 × 104 to 7.0 × 104, the EE and LC of lysozyme were both increased. This was because when the Mv of CS with the same DD increased, every chain of CS had more amino groups to interact with TPP. In the process of chains of chitosan entangling with TPP to form particles, longer chains made the size of nanoparticles formed larger. Accordingly, the encapsulation of lysozyme was increased when increasing the Mv of CS (Xu & Du, Citation2003).
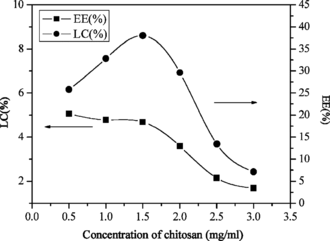
Effect of chitosan concentration in encapsulation of lysozyme
shows that the change of EE with increased CS concentration contained two steps. Step (1): increasing CS concentration from 0.5 to 1.5 mg/ml increased EE from 25.8% to 38.0%. This was because there were more CS-TPP nanoparticles formed as the amount of CS increased, and the amount of lysozyme entrapped in the nanoparticles was also increased. Step (2): increasing CS concentration from 1.5 to 3.0 mg/ml decreased EE from 38.0% to 7.1%. Xu and Du (Citation2003) stated that lower concentration of CS with lower viscosity promoted encapsulation of bovine serum albumin (BSA) in the study of CS-TPP nanoparticles. The viscosity of CS became too high as the concentration of CS further increased, and it made the encapsulation of lysozyme more difficult because the increased viscosity might hinder lysozyme to diffuse in the chitosan solution, so the EE was decreased.
However, increasing CS concentration from 0.5 to 3.0 mg/ml decreased the LC slightly. This indicated that the concentration of chitosan had little effect on entrapping the lysozyme into the nanoparticles.
The CS-TPP nanoparticles were once reported that they had a high affinity for the association of certain macromolecules, such as BSA (Calvo et al., Citation1997) and tetanus toxoid (Vila et al., Citation2004). For example, the LC of BSA in CS-TPP nanoparticles could be up to 50%, and the LC was enhanced as the concentration of chitosan increased. This was because the isoelectric point of BSA was 4.8, while the pH of nanoparticles formation medium was higher than the isoelectric point of BSA, BSA was negatively charged and interacted with the protonized amine groups of chitosan, thus leading to the entrapment of high amount of BSA in the nanoparticles, further the high LC (Janes et al., Citation2001). However, the isoelectric point of lysozyme here was 10.5, lysozyme was positively charged at the pH of nanoparticles formation medium (pH 4.5–5.0). The chitosan in the acid solution was positively charged, too, thus lysozyme and chitosan did not have an electrostatic interaction in the nanoparticles formation medium as BSA did. The mechanism of lysozyme entrapped into CS-TPP was a controlled gelation process, just like Fernandez-Urrusuno and his co-workers (Citation1999) interpreted why insulin could be associated with CS nanoparticles even at a pH where insulin was predominantly positively charged. Accordingly, the LC of lysozyme in the CS-TPP here was much lower than that of the negatively charged macromolecules in the CS-TPP nanoparticles; the LC of lysozyme declined as the concentration of chitosan increased, contrary to that of BSA.
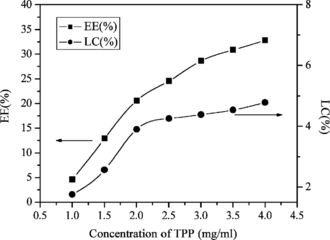
Effect of TPP concentration in encapsulation of lysozyme
As shown in , when the concentration of TPP increased, the EE and LC of lysozyme were increased. Higher concentration of TPP could gelate a greater amount of CS, and so a relatively greater amount of lysozyme could be entrapped in nanoparticles.
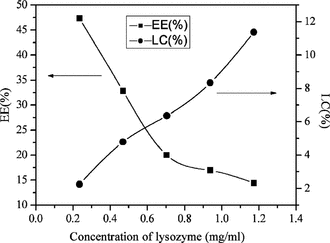
Effect of lysozyme initial concentration in encapsulation of lysozyme
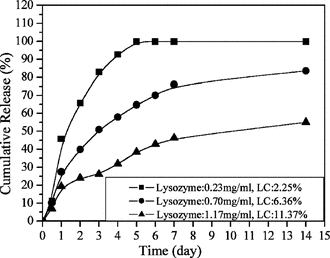
As shown in , increasing the lysozyme initial concentration from 0.23 to 1.17 mg/ml increased the LC from 2.3% to 11.4% but decreased the EE from 47.3% to 14.4%.
The growing LC and high EE could be easily explained by the method of nanoparticle preparation. TPP aqueous was added to a chitosan solution containing lysozyme, and the chitosan formed gel nanoparticles instantaneously entrapped the lysozyme in a three-dimensional lattice of ionically crosslinked chitosan (Lemoine et al., Citation1998). Increasing the initial amount of lysozyme in the chitosan solution increased the amount of lysozyme in the lattice and therefore the lysozyme loading. However, the EE was decreased, which suggested that the quantity of polymer present became insufficient to entrap the total amount of protein.
In vitro. release of lysozyme from the nanoparticles
showed the behavior of lysozyme release from chitosan nanoparticles in vitro.. All samples exhibited a two-release stage: a rapid release over the first 2 days followed by a relatively slow release. However, the release rate of all samples were different from each other. Consequently, these results indicated that there were possibilities of modulating the release rate of lysozyme by adjusting the parameters of chitosan, lysozyme, and TPP.
Two processes could explain the release of a drug from a particle: diffusion and erosion. Lysozyme could diffuse out of the chitosan nanoparticles as the TPP-chitosan matrix swelled (Ko et al., Citation2002), following the water phase that filled the matrix of the nanoparticles. Lysozyme could also release from the chitosan nanoparticles through the erosion of the matrix as the chitosan backbone hydrogradated into smaller molecular weight components. The burst release of lysozyme in the first 2 days was associated with those protein molecules dispersing close to the nanoparticle surface, which easily diffused out in the initial incubation time, whereas the second stage might correspond with those lysozyme more efficiently entrapped in the nanoparticles, which released from the nanoparticles by diffusion and erosion (Zhou et al., Citation2001).
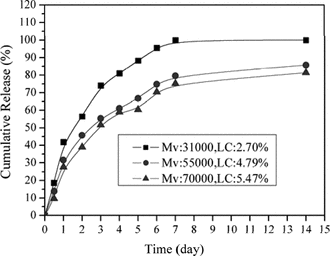
Effect of chitosan Mv in release in vitro
shows the release rate of lysozyme decreased as the Mv of CS increased. Okhamafe and Goosen (Citation1993) stated that permeability of coacevate microcapsule was lower with increasing Mv due to increased chain packing and rigidity, as well as increased interchain bonding. The theory was helpful to this situation. The permeability of the nanoparticles was lower as the Mv of CS increased; as a result, increased Mv of CS provided a decreased release rate of lysozyme.
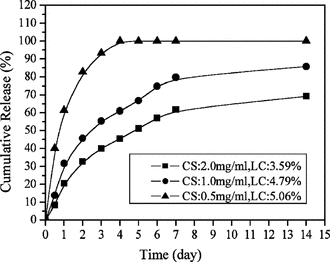
Effect of chitosan concentration in release in vitro
shows that as the concentration of CS increased, the release rate of lysozyme from nanoparticles decreased. Lim and his co-workers (Citation1997) had studied the effect of CS concentration on the permeability of microspheres of CS-TPP; they stated that increasing the concentration of chitosan resulted in the increased viscosity of chitosan solution, and the increased viscosity of chitosan solution formed relatively strong walls of microspheres upon interaction with TPP. High crosslinking density of CS-TPP matrix resulted in less swelling ability, therefore the release of lysozyme decreased. Accordingly, the release rate of lysozyme decreased as the amount of CS in the nanoparticles increased.
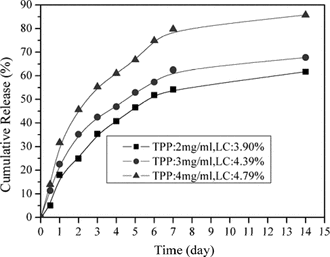
Effect of TPP concentration in release in vitro
As shown in , the release rate of lysozyme increased as the amount of TPP increased. This was because higher amount of TPP provided higher LC of lysozyme, and further the faster release rate of lysozyme. Usually, the lysozyme release rate was highly influenced by the amount of protein entrapped. The high LC provided a fast release rate, which was attributed to lysozyme concentration gradient.
Effect of initial lysozyme concentration in release in vitro
As shown in , the release rate of lysozyme decreased as the initial lysozyme concentration increased. Cumulative release was calculated with following equation:
which meant the release rate was both influenced by the amount of release lysozyme and the total amount of lysozyme entrapped. Although larger amount of lysozyme was released from the nanoparticles with higher loading content, the total amount of lysozyme entrapped had more influence on the release rate than the amount of released lysozyme. Accordingly, the nanoparticles with higher LC of lysozyme showed a lower release rate of lysozyme.
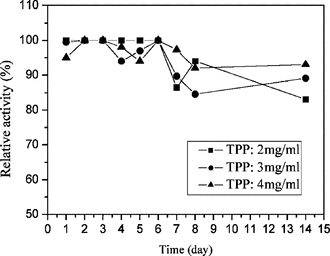
Relative activity of released lysozyme from the nanoparticles in vitro
shows influence of TPP on the RA of lysozyme. The result showed that the concentration of TPP ranging from 1 to 4 mg/ml on the acid condition (pH 4.5–5.0) had only a little effect on the RA of lysozyme, and all the samples in vitro. release kept above 85% RA. Lysozyme aqueous solution was reported to be very stable on acid condition, but on alkaline condition the structure of lysozyme was easily destroyed. The pH of TPP with concentration ranging from 1 to 4 mg/ml ranged from 9.0 to 9.8, so lysozyme would lose activity when TPP aqueous solution was added to lysozyme aqueous solution. However, on the condition of nanoparticles formation medium, the pH was maintained between 4.5 and 5.0. Accordingly, this condition of nanoparticle formed was good to lysozyme.
Conclusions
Lysozyme-loaded CS-TPP nanoparticles were prepared by an ionic gelation method. They were spherical in shape and 50–280 nm in diameter. Several parameters including Mv of CS, content of CS, TPP, and initial lysozyme were investigated in the EE, LC, release and activity of lysozyme in chitosan nanoparticles. All the parameters had a large effect on the EE of lysozyme but little on the LC. Higher Mv of chitosan made higher EE, LC of lysozyme but lower release rate of lysozyme. Increasing CS content made the EE first enhanced then declined, the LC decreased, and the release rate declined. Higher content of TPP made a higher EE, LC of lysozyme and increased the release of lysozyme. Increasing initial lysozyme concentration decreased EE but increased LC and decreased the release. The condition of lysozyme-loaded nanoparticles formed was good to lysozyme. As a result, relative parameters can be modulated to satisfy the needs of protein delivery for certain usage. Further study will focus on the application of these lysozyme-loaded chitosan nanoparticles to accelerate the degrading rate of the biodegradable polymer.
Acknowledgements
Financial support from High Technology Program of National Science and Technology Department of China (973 Program, grant no. G1999054306) and Guangdong Provincial Natural Science Foundation of China (grant no. 32204056) are gratefully acknowledged.
References
- Calvo P, Remuñán-López C, Vila-Jato JL, Alonso MJ (1997): Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci 63: 125–132. [CSA]
- Fernandez-Urrusuno R, Calvo P, Remuñán-López C, Vila-Jato JL, Alonso MJ (1999): Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm Res 16: 1576–1581. [INFOTRIEVE], [CSA], [CROSSREF]
- Haque T, Chen H, Ouyang W, Martoni C, Lawuyi B, Urbanska A, Prakash S (2005): Investigation of a new microcapsule membrane combining alginate, chitosan., polyethylene glycol and poly-L-lysine for cell transplantation applications. Int J Artif Organs 28: 631–637. [INFOTRIEVE], [CSA]
- Janes KA, Alonso MJ (2003): Depolymerized chitosan nanoparticles for protein delivery: Preparation and characterization. J Appl Polym Sci 88: 2769–2776. [CSA], [CROSSREF]
- Janes KA, Calvo P, Alonso MJ (2001): Polysaccharide colloidal particles as delivery systems for macromolecules. Adv Drug Deliv Rev 47: 83–97. [INFOTRIEVE], [CSA], [CROSSREF]
- Ko JA, Park HJ, Hwang SJ, Park JB, Lee JS (2002): Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int J Pharm 249: 165–174. [INFOTRIEVE], [CSA], [CROSSREF]
- Kurita K, Kaji Y, Mori T, Nishiyama Y (2000): Enzymatic degradation of β-chitin: Susceptibility and the influence of deacetylation. Carbohyd Polym 42: 19–21. [CSA], [CROSSREF]
- Lee YC, Yang D (2002): Determination of lysozyme activities in a microplate format. Anal Biochem 310: 223–22. [INFOTRIEVE], [CSA], [CROSSREF]
- Lemoine D, Wauters F, Bouchend'homme S, Préat V (1998): Preparation and characterization of alginate microspheres containing a model antigen. Int J Pharm 176: 9–19. [CSA], [CROSSREF]
- Lim LY, Wan LSC, Thai PY (1997): Chitosan microspheres prepared by emulsification and ionotropic gelation. Drug Dev Ind Pharm 23: 981–985. [CSA]
- Mi FL (1999): Chitosan-polyelectrolyte complexation for the preparation of gel beads and controlled release of anticancer drug. II. Effect of pH-dependent ionic crosslinking or interpolymer complex using tripolyphosphate or polyphosphate as reagent. J Appl Polym Sci 74: 1093–1107. [CSA], [CROSSREF]
- Okhamafe AO, Goosen MFA (1993): Control of membrane permeability in microcapsules. In: Goosen MFA, ed., Fundamentals of Animal Encapsulation and Mobilization. Boca Raton, Florida, CRC Press Inc., 1993, pp. 55–78.
- Peng J, Xing X, Wang K, Tan W, He X, Huang S (2005): Influence of anions on the formation and properties of chitosan.-DNA nanoparticles. J Nanosci Nanotechnol 5: 713–717. [INFOTRIEVE], [CSA], [CROSSREF]
- Smith JS, Bedrov D, Smith GD (2003): A molecular dynamics simulation study of nanoparticles interactions in a model polymer-nanoparticle composite. Compos Sci Technol 63: 1599–1605. [CSA], [CROSSREF]
- Samuels RJ (1981): Solid-state characterization of the structure of chitosan films. J Polym Sc: B Polym Phys 19: 1081–1105. [CSA], [CROSSREF]
- Vila A, Sánchez A, Janes K, Behrens I, Kissel T (2004). Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice. Eur J Pharm Biopharm 57: 123–131. [INFOTRIEVE], [CSA], [CROSSREF]
- Williams DF (2005): To engineer is to create. Presentation. The 8th Annual Meeting of Tissue Engineering Society International, Shanghai.
- Wu JP (2003): Improved method for acid-base titration determination of the degree of N.-acetylation of chitosan. J Beij Un Univ (Nat Sci) 17: 52–57. [CSA]
- Wang W (1991): Determination of the Mark-Houwink equation for chitosans with different degrees of deacetylation. Int J Biol Macromol 13: 281–285. [INFOTRIEVE], [CSA], [CROSSREF]
- Xu YM, Du YM (2003): Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int J Pharm 250: 215–226. [INFOTRIEVE], [CSA], [CROSSREF]
- Xia WS, Su C (2002): The reaction mechanism and kinetics of chitin/chitosan degradation with hydrolyses. J Wux Univ Light Ind 21: 434–438. [CSA]
- Zhou SB, Deng XM, Li XH (2001): Investigation on a novel core-coated microspheres protein delivery system. J Controlled Release 75: 27–36. [CSA], [CROSSREF]