Abstract
The neutralizing effects of methanol extracts of Indigofera pulchra. Willd (Papilionaceae), Aristolochia albida. Duch (Aristolochiaceae), Guiera senegalense. J.F.Gmel (Combretaceae), and Sterculia setigera. K. Schum (Sterculiaceae) were investigated to validate traditional claims of usefulness of the plants in management of poisonous snakebites. Extracts of Indigofera pulchra. and Aristolochia albida. gave 33.3% and 44.4% protection to mice treated with minimum lethal dose of venom; some gross pathologic symptoms of envenomation were alleviated. However, minimal activities were shown by Guiera senegalense. and Sterculia setigera.. Both Indigofera pulchra. and Aristolochia albida. were found to neutralize the anticoagulant, hemolytic, and phospholipase activity of crude venom. This study showed that Indigofera pulchra. and Aristolochia albida. are useful in some pathologic effects of Naja nigricollis. Broadley (Elapidae) venom, and this provides some scientific basis for the use of the plants in management of poisonous snakebites.
Keywords:
Introduction
Poisonous snakebites in Northern Nigeria are usually due to several types of snakes. The snakes particularly responsible for serious medical emergencies are the Naja. species [mainly by Naja nigricollis. Broadley (Elapidae) and Naja katiensis. Broadley & Hallowell (Elapidae)], and less commonly by Naja melanoleuca. Rödel & Mahsberg (Elapidae)and Naja mossambica. Broadley & Hallowell (Elapidae), the Echis. species [Echis ocellatus. Schneider (Viperidae) and Echis coloratus. Schneider (Viperidae)] and Bitis. species [principally Bitis arietans. Rödel & Mahsberg (Viperidae), Bitis nasicornis. Rödel & Mahsberg (Viperidae), and Bitis gabonica gabonica. Mcdiarmid, Campbell & Touré (Viperidae)] (Pugh et al., Citation1980; Warrel, Citation1989).
Envenomation by the Naja spp.. can cause severe local symptoms that include swelling, blistering, and necrosis with general symptoms of systemic envenomation; neurotoxicity is common. The Hausa and Fulani tribes of northern Nigeria have several claims of effective use of plants in treatment of poisonous snakebites including bites due to Naja nigricollis., the most common of the spitting cobras (Haruna & Choudhury, Citation1995; Abubakar et al., Citation2000). Personal contact with several traditional medical practitioners in some snake endemic areas of northern Nigeria (particularly Zaria, Malumfashi, and Kaltungo) have shown that the plants used in many of their prescriptions for treatment of snakebites are similar and sometimes related to the type of snakes found in these areas. The plant treatments are particularly popular, especially for prophylaxis of snakebites during the rainy season when the incidence of snakebites is usually high.
Snake venom antiserum development and standardization is expensive and requires ideal storage conditions (Theakson et al., Citation2003); storage facilities for antiserum may be lacking in the usually remote snake endemic areas of Africa. The use of plants as alternatives for treatment of poisonous bites is important in remote areas where there is no accessibility to hospitals and storage facilities for antivenom. Plants are popular alternatives for treatment of poisonous snakebites (Nuno et al., Citation1994; Selvanayagam et al., Citation1995). Some of these traditional methods have a scientific basis (Abubakar et al., Citation2000; Alam & Gomes, Citation2003); quite a number of antivenom compounds have been isolated from some of these plants (Tsai et al., Citation1980; Haruna & Choudhury, Citation1995; Mors et al., Citation2000).
This communication reports the in vitro. and in vivo. neutralizing effect of some plant extracts against the venom of Naja nigricollis., one of the snakes responsible for quite a number of fatal bites in northern Nigeria.
Materials and Methods
Snake and venom collection
Naja nigricollis. (West African black spitting cobra) were captured from Zaria and environments in Nigeria, identified by taxonomic means, and specimens were kept at the Department of Pharmacognosy and Drug Development, Faculty of Pharmaceutical Sciences, Ahmadu Bello University, Zaria, Nigeria. Venom was collected by the milking method (Markfarlane, Citation1967) from 10 adult Naja nigricollis. of both sexes, pooled, and lyophilized and stored at −4°C until required. This was subsequently referred to as the crude venom.
Animals
Locally bred adult Swiss-strain albino mice of both sexes, weighing 25–33 g were used. The animals were fed standard mouse pellets, and water was supplied ad libitum.. They were allowed to acclimatize before the tests. The animal care and handling were conducted in compliance with the National Regulations for Animal Research. The university ethical committee reviewed the protocols, which are consistent with the International Animal Welfare Guidelines.
Plant collection and preparation
The plant materials were collected based on their ethnomedical uses against poisonous snakebites and authenticated at the Herbarium of Department of Biological Sciences, Faculty of Science, Ahmadu Bello University (Zaria, Nigeria) where voucher specimens of these plants were kept. The plant materials collected were Indigofera pulchra. (aerial part), Aristolochia albida. (rhizomes), Sterculia setigera. (bark), and Guiera senegalense. (leaves). These were air-dried, powdered, and 500 g of each plant part was percolated with 95% methanol, the solvent was evaporated in vacuo. to yield syrupy residue referred to as extracts, which was stored desiccated until required for use. Appropriate stock dilutions of the extracts were made in normal saline, centrifuged at 2000 rpm for 10 min at room temperature and, subsequently, the supernatants were used in venom neutralization studies.
Neutralization of lethal effect of crude venom
The minimum lethal dose (MLD) of venom after intraperitoneal (i.p.) injection was as previously reported (Abubakar et al., Citation2000). A preliminary screening for minimum tolerated dose (dose that starts producing toxic symptoms) was determined for the various extracts; in this experiment we found a convenient i.p. dose of 100 mg kg−1 to be devoid of any apparent toxicity for the extracts. This dose was selected for evaluation of in vivo. antivenom activity. However, in traditional medicine of the Hausa and Fulani (two local tribes of northern Nigeria), doses are usually oral, not standardized, and dependent on the type of bites.
The effect of extract was determined by pretreating a group of mice (n = 9) with an i.p. dose of 100 mg kg−1 of the various extracts followed 1 h later by an i.p. injection of MLD venom. The average time and number of deaths were recorded within 24 h. Gross pathologic examination was performed on the brain, liver, kidneys, alimentary tract, and spleen of all dead mice. Mice that survived this experiment were euthanatized at the end of the study and were similarly examined.
Neutralization of anticoagulant activity of crude venom
This was performed by determining the effect on recalcification times of citrated bovine plasma using the method of Theakston and Reid (Citation1983). Citrated bovine plasma (200 µl) was incubated in a water bath at 37°C. To each sample, 10 µl of dilution of crude venom was added, the mixture was then diluted with 100 µl phosphate-buffered saline (PBS), pH 7.0. Finally, 10 µl of 25 mM of CaCl2 was added, and the recalcification time was recorded.
Dilutions of 1 mg ml−1 of extracts were made from the stock in PBS (the extracts at this dose do not affect the apparent coagulability of the citrate bovine plasma). The effect of extracts was determined by replacing the PBS above with the extract dilution in PBS. The experiment was carried out for venom dilutions of 0.001, 0.01, and 0.1 µg. The experiment was carried out in replicates (n = 5) and recalcification times given as mean ± SD.
Neutralization hemolytic activity of crude venom
Bovine erythrocytes were used to determine the hemolytic activity. Erythrocytes were washed with saline (0.9%) by centrifugation (2000 rpm) for 10 min. After repeated washings with saline, a 1% cell suspension was prepared. Tubes with the venom dilutions (equivalent to 10 µg), mixed with 10 ml of 1% cell suspension in saline were incubated at 37 ± 1°C for 60 min. The reaction was stopped by adding 3 ml of chilled PBS. The mixtures were centrifuged at 2000 rpm for 10 min, and absorbance of the supernatant was measured at 540 nm. Supernatants treated with 3 ml of distilled water were taken as 100% lysis. The effect of the extract was determined by mixing venom dilution with 0.1 ml of extract dilutions (1 mg ml−1) in saline before adding to the cell suspension, and the supernatant of a tube with 3 ml of distilled water containing 0.1 ml of extract dilution (1 mg ml−1) in distilled water) was taken as 100% lysis. The experiment was carried out in replicates (n = 5) and percent hemolysis given as mean ± SD.
Neutralization of phospholiphase A2 (PLA2) activity of crude venom
The phospholipase A2 activity of venom was determined by a modified egg yolk coagulation method of Habermann and Neumann (Citation1954). Briefly, fresh egg yolk was homogenized in distilled water to yield a concentration of 100 mg ml−1. The venom (10 µl) and 10 µl of 50 mM Tris/HCl buffer (pH = 8.0) were incubated with 1000 µl substrate at 37 ± 1°C. At the end of 10 min incubation, immersing the mixture in boiling water for 2 min stopped the reaction. The liberated fatty acids were titrated against 20 mM NaOH using phenolphthalein as indicator. The effect of extract was determined by replacing the 10 µl of 50 mM Tris/HCl buffer above with 10 µl of dilution of the extract in 50 mM Tris/HCl buffer (1 mg/10 µl), incubated for 60 s before adding to the substrate. The dose relationship was determined by incubating the dilution equivalent to 1 mg of venom with the following doses of extract: 1, 10, and 100 mg. The phospholipase A2 activity was given as the number of moles of acid (average of five determinations ± SD) liberated by 1 mg of venom in 1 h.
Results and Discussion
Plant collection and identification
The plants listed in are used for treatment of poisonous snakebites in Hausa and Fulani ethnomedicine. This information was collated from traditional medical healers, snake charmers, farmers, and nomads around Zaria, Malumfashi, and Kaltungo areas representing a wide distribution of ethnomedical practice within the geographic zone of northern Nigeria. The method and preparation of these plants as shown in is related to the type of snakes found in these areas. It is important to note that the number of plants used in these practices is inexhaustible; some of the plants are taken as prophylaxis of snakebites, especially during the onset of the rainy season when snakebites are common (Abubakar et al., Citation2000). Naja nigricollis. is commonly found within these areas and may be responsible for quite a number of human encounters and fatal bites (Pugh & Theakston Citation1980; Pugh et al., Citation1980)
Table 1. Plants used in poisonous snakebites in northern Nigeria.
Effect of extract on lethal dose (MLD) of the crude venom
Mice treated with MLD of venom showed excitement followed by depression, paralysis (especially of the hind limb), and death usually accompanied these symptoms. These are classical symptoms of neurotoxicity. The plants are usually taken orally in snakebites; however, we used the i.p. route to evaluate antivenom effect because it is more convenient and produces rapid and reproducible results (Abubakar et al., Citation2000). shows the effect of 100 mg kg−1 extracts against the minimum lethal dose of venom. Survival % (protection) calculated over 24 h showed extracts of Indigofera pulchra. and Aristolochia albida. to have offered the highest protection (33.3% and 44.4%, respectively), as compared with Guiera senegalense. and Sterculia setigera. (22.2% and 0%, respectively) The average time of death was significantly increased by extracts of Indigofera pulchra, Aristolochia albida., and Guiera senegalense. (p < 0.05 using one-way ANOVA). The extracts prolong the time of death, presumably due to some active constituent. Plant constituents are known to neutralize venom component in vivo. (Mors et al., Citation2000). This may also be responsible for the observed reduction in the enlargement and congestion in the lungs, spleen, liver, and the kidneys. However, hemorrhage in these organs was not much different from those in venom-treated mice. It was found that Sterculia setigera. does not have a significant effect on the MLD of venom. The results show the extracts of Indigofera pulchra, Aristolochia albida., and Guiera senegalense. can ameliorate the lethal effect of Naja nigricollis., and this effect is dose-dependent.
Table 2. The effect of 100 mg kg−1 i.p. dose of some plant extract on the lethal properties of Naja nigricollis. venom.
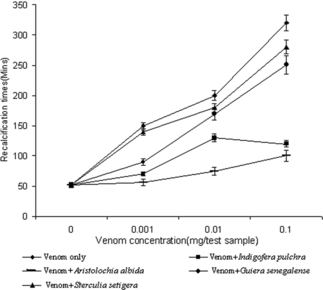
Effect of crude venom on recalcification times of citrated bovine plasma
The crude venom produces a dose-dependent increase in the recalcification times of bovine plasma (between 0.001 and 0.1 µg/200 µl of plasma). At concentrations of venom greater than 1 µg (strong anticoagulant effect), coagulation times are greater than 20 min and mostly with poorly formed clots. shows the effect of a single dose (1 mg) of different plant extracts on the different dilutions of venom. Both extracts of Indigofera pulchra. and Aristolochia albida. reduced the recalcification time significantly (p < 0.05 using one-way ANOVA). This was not, however, observed for Guiera senegalense. and Sterculia setigera.. It may be speculated that the inhibitory effect is due to a wide range of compounds, mostly phenolic compounds in these plants (flavonoids and tannins). It is known that phenolic compounds can precipitate proteins (Harbone, Citation1973) leading to their inactivation and removal (Okonogi et al., Citation1979).
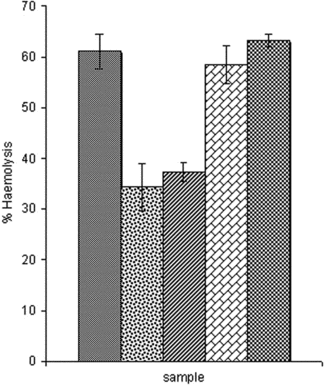
Hemolytic activity of crude venom
The crude venom produces hemolysis of the bovine red blood cells. shows the hemolytic property of the crude venom and the effect of single concentrations of plant extracts. The venom-induced hemolytic activity was significantly reduced by the extracts of Indigofera pulchra. and Aristolochia albida. and not by Guiera senegalense. and Sterculia setigera. extracts. Blood hemolysis by venoms is mostly due to the phospholipase enzymes (Salach et al., Citation1971; Teng et al., Citation1986).
Figure 2 Effect of extracts on the hemolytic properties of venom of Naja nigricollis.. Bars represent the mean ± SD. The difference between hemolytic activity of venom and venom incubated with extract is statistically significant for Indigofera pulchra. and Aristolochia albida. (at p < 0.05; one-way ANOVA). ![]()
Phospholipase activity of crude venom
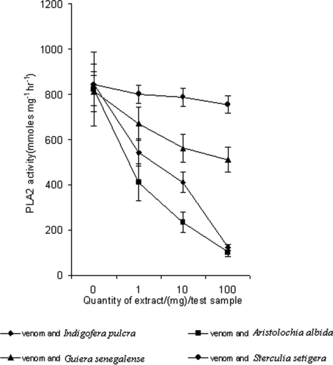
Phospholipases are enzymes that perform a highly regulated role in lipid metabolism by the stereospecific hydrolysis of 3-sn.-phosphoglycerides. The phospholipase activity of crude venom and venom in the presence of extracts is shown in . The PLA2 activity of crude venom was found to be 852.0 ± 26.2 µmoles mg−1 h−1: this activity was reduced by extracts of Indigofera pulchra, Aristolochia albida., and Guiera senegalense. significantly (at (p < 0.05), but not with the extract of Sterculia setigera..
Table 3. The effect of some plant extracts on the phospholipase A2 activity of crude Naja nigricollis. venom.
shows the effect of different concentrations of the extracts at a single test dose of venom. The effect of Indigofera pulchra, Aristolochia albida., and Guiera senegalense. was dose-dependent. Because the venom is incubated with the extract prior to addition to substrate, the observed reduction in PLA2 activities may be as a result of interaction of amino acid in the catalytic site with the substrate. Our recent report showed that columbin, an active constituent of Aristolochia albida., noncompetitively inhibits PLA2 from Naja nigricollis. (Nok et al., Citation2002). The current observation, the reduction of PLA2 activity, suggests the presence of some active ingredients in the plants that interact with the enzyme.
Figure 3 Effect of different doses of some plant extract on the phospholipase A2 activity of crude Naja nigricollis. venom. The extracts of Indigofera pulchra, Aristolochia albida., and Guiera senegalense. significantly reduced the PLA2 activity of crude venom (at p < 0.05; one-way ANOVA).
Phospholipase enzymes from elapid venoms are known to be responsible for neurotoxicity and myotoxicity leading to complete failure of nerve functions and rapid degeneration of individual muscle, respectively (Salach et al., Citation1971; Ouyang & Huang, Citation1984). Inhibition of PLA2 activity by the extract may be responsible for alleviation of these symptoms, including hemolysis of blood due to these pathogenic enzymes.
Conclusions
The extracts of Indigofera pulchra. and Aristolochia albida. have some beneficial effects on some toxic effects of venom. Thus, the use of these plants in Hausa and Fulani ethnomedicine may have some scientific basis.
Acknowledgment
The authors wish to acknowledge the Ahmadu Bello University Board of Research for funding part of this work.
References
- Abubakar MS, Sule MI, Pateh, UU, Abdurahman EM, Haruna AK, Jahun BM (2000): The in vitro. snake venom detoxifying action of the leaf extract of Guiera senegalensis.. J Ethnopharmacol 69: 253–257. [INFOTRIEVE], [CSA]
- Alam MI, Gomes A (2003): Snake venom neutralization by Indian medicinal plants (Vitex negundo. and Emblica officinalis.) root extracts. J Ethnopharmacol 86: 75–80. [INFOTRIEVE], [CSA], [CROSSREF]
- Habermann E, Neumann W (1954): Egg yolk coagulation method. Physiol Chem 297: 174–176. [CSA]
- Harbone JB (1973): Phytochemical Methods, 2nd ed. London, Chapman and Hall, pp. 213–216.
- Haruna AK, Choudhury MK (1995): In vivo. antisnake venom acivity of the furoid diterpene from Aristolochia albida. Duch. Indian J Pharm Sci 27: 222–224. [CSA]
- Markfarlane RG (1967): Russell's viper venom, 1953–1964. Br J Haematol 13: 437–451. [CSA]
- Mors WB, Celia do Nascimento M, Ruppelt Pereira BM, Alvarez Pereira N (2000): Plant products active against snake bite—the molecular approach. Phytochemistry 55: 627–642. [INFOTRIEVE], [CSA], [CROSSREF]
- Nok AJ, Balogun E, Lori JA, Abubakar MS (2002): Inhibition of Naja nigricollis. venom acidic phospholipase A2 catalysed hydrolysis of ghost red blood cells by columbin. J Enzyme Inhibit Med Chem 17: 55–59. [CSA], [CROSSREF]
- Nuno AP, Ruppelt Pereira BM, Celia do Nascimento M, Paz Parente J, Mors WB (1994): Pharmacological screening of plants recommended by folk medicine as snake venom antidotes; IV. Protection against Jararaca. venom by isolated constituents. Planta Med 60: 99–100. [CSA]
- Okonogi T, Hattori Z, Ogiso A, Mitsui S (1979): Detoxification by persimmon tannin of snake venom and bacterial toxins. Toxicon 17: 524–527. [INFOTRIEVE], [CSA], [CROSSREF]
- Ouyang C, Huang TF (1984): Effect of the purified phospholipases A2 from snake and bee venoms on rabbit platelet function. Toxicon 22: 705–718. [INFOTRIEVE], [CSA], [CROSSREF]
- Pugh RNH, Theakston RDG (1980): Incidence and mortality of snake bite in savanna Nigeria. Lancet 2: 1181–1183. [INFOTRIEVE], [CSA], [CROSSREF]
- Pugh RNH, Theakston RDG, Reid HA, Bhar SI (1980): Malumfashi endemic disease project XIII: Epidemology of human encounters with the spitting cobra (Naja nigricollis.) in Malumfashi area of Northern Nigeria. Ann Trop Med Parasitol 74: 523–530. [INFOTRIEVE], [CSA]
- Salach JI, Turini P, Seng R, Hauber J, Singer T (1971): Phospholipase A of snake venoms I. Isolation and molecular properties of isoenzymes from Naja naja. and Vipera russellii. venoms. J Biol Chem 246: 331–336. [INFOTRIEVE], [CSA]
- Selvanayagam ZE, Gnavavendhan SG, Balakrishna K, Rao RB, Ali SU (1995): Survey of medicinal plants with antisnake venom activity in Chengalpattu District, Tamilnadu, India. Fitoterapia 66: 488–494. [CSA]
- Teng CM, Kuo YP, Lee LG, Ouyang C (1986): Effect of cobra venom phospholipase A2 on platelet aggregation in comparison with those produced by arachidonic acid and lysophophatidylcholine. Thromb Res 44: 875–886. [INFOTRIEVE], [CSA], [CROSSREF]
- Theakson RDG, Reid HA (1983): The development of simple standard assay procedures for characterization of snake venoms. Bull WHO 61: 949–956. [CSA]
- Theakston RDG, Warrell DA, Griffiths E (2003): Report of a WHO workshop on the standardization and control of antivenoms. Toxicon 41: 41–557. [CSA], [CROSSREF]
- Tsai H, Yan LL, Chang C (1980) : Inactivation of Formosan snakes venom in vivo. by aristolochic acid the chemical component of Aristolochia radix.. Tai-wan Ko' Hsueh 34((2)): 40–44 (Taiwanese). [CSA]
- Warrell DA (1989): Snakebites in five continents. In: Bunch C, ed., Horizon of Medicine. New York, Baillier Tindal, pp. 106–114.