Abstract
Root cultures of Senna alata. (L.) Roxb. were established in liquid B5 medium, supplemented with 0.5 mg l−1 α.-naphthalene acetic acid (NAA) and 1.0 mg l−1 kinetin. The root cultures, which were established from the high-anthraquinone-producing plants, accumulated higher amounts of emodin and chrysophanol than those established from the low-anthraquinone-producing plants and leaves and roots of the intact plants.
Introduction
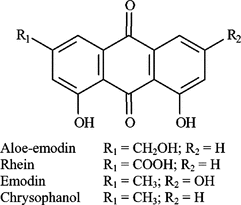
Senna alata. (L.) Roxb. or Cassia alata. L. (Leguminosae) is a shrub that has long been traditionally used for the treatment of constipation and dermatophyte infections (Farnsworth & Bunyapraphatsara, Citation1992). Anthraquinone glycosides were demonstrated as the active constituents for the laxative properties (Elujoba et al., Citation1989) and the aglycones, including aloe-emodin, rhein, emodin, and chrysophanol (), as antifungal active agents (Agarwal et al., Citation1976, 2000; Zhou et al., Citation2006).
Plant tissue cultures provide an alternative method of secondary metabolite production, instead of using the whole plant or total chemical synthesis. The use of plant cell cultures as an alternative source of plant constituents has a number of advantages, in particular, independence from geographical, climatic, and political environments (Charlwood & Rhodes, Citation1990). Until now, an establishment of an in vitro. culture of S. alata. in order to study anthraquinone production has not been reported. As part of our interest in the use of tissue cultures as an alternative source of plant chemicals, the root cultures of S. alata. were established. In this study, selection of high-yielding plants and medium manipulation were examined in order to increase anthraquinone production. A high-performance liquid chromatography (HPLC) system for quantitative analysis of each anthraquinone was developed.
Materials and Methods
Preparation of S. Alata. plantlets
S. alata. seeds were collected from Songkhla Province, Thailand. The sterile seeds were transferred to hormone-free B5 basal solid medium and incubated at 25 ± 2°C with a 16-h daily light period for 2 months.
Selection of high-yielding plants
Preparation of S. alata. leaf extracts
Fifty-six leaf samples were separately collected from S. alata. plantlets (2 months old). The leaves were dried at 50°C for 24 h, and the dried powdered leaves (5 mg) were extracted with methanol (10 ml) under reflux for an hour. The extracts were filtered, evaporated to dryness and adjusted to the volume of 1 ml with methanol. The contents of rhein and aloe-emodin were determined by HPLC. All sample analyses were carried out in triplicate.
Quantitative analysis of anthraquinone content
HPLC analysis was carried out using a Waters 600 pumps equipped with a Waters 966 photodiode-array detector (PDA) and autosampler. Data analysis was performed using Millenium 2.0 software (Waters, Milford, USA). Separation was achieved isocratically at 25°C on a 150 mm × 4.6 mm i.d. TSK-gel ODS-80Ts column. The mobile phase consisted of 2% aqueous acetic acid–methanol (gradient from 55% methanol to 100% methanol in 60 min) and was pumped at a flow rate of 1 ml/min. The injection volume was 20 µl. The quantitation wavelength was set at 254 nm. The areas under the peaks were converted to concentration by using their calibration curves. The calibration curves of rhein, aloe-emodin, emodin, and chrysophanol were established from authentic samples. The linearity of the chrysophanol calibration curves was observed in the range from 1.6 to 200 µg/ml and aloe-emodin, emodin, and rhein from 0.3 to 40 µg/ml with r2 more than 0.999 for all the compounds tested. Each calibration point was carried out in triplicate.
Establishment of S. alata. root cultures
S. alata. root cultures were initiated from the roots of the plantlets that were selected from either high-anthraquinone-producing plantlets or nonselected plantlets. The root cultures were grown in 250-ml Erlenmeyer flasks containing 50 ml of liquid B5 medium supplied with 0.5 mg l−1 NAA, 1.0 mg l−1 kinetin, and 2% w/v sucrose. The flasks were incubated on a rotary shaker at 25°C, 90 rpm with a 16-h light. The tissue cultures were maintained under these conditions and subcultured to fresh medium every 4 weeks.
Determination of anthraquinone formation in S. alata. cultures
The root cultures (1 month old), from different culture media, were separately harvested by vacuum filtration. The harvested biomasses were dried in a hot air oven at 50°C and ground. The powdered sample (0.2 g) was extracted with 20 ml of methanol under reflux for an hour and filtered. After the filtrates were evaporated to dryness, the obtained residues were dissolved in methanol and adjusted to volume in a 10-ml volumetric flask. These sample preparations were then subjected to analysis of anthraquinone accumulation using HPLC analysis as described above.
Results and Discussion
Selection of anthraquinone high-yielding plants
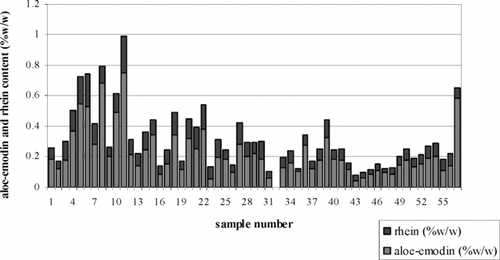
A variation of aloe-emodin and rhein content in S. alata. leaves was assessed in 56 plantlet samples. The average yields of rhein and aloe-emodin in the samples were 0.09 ± 0.047% and 0.21 ± 0.151% w/w, respectively. The content of aloe-emodin was usually higher than rhein. Nevertheless, some of them contained aloe-emodin and rhein content higher than the natural intact plants (). The top five plantlets, which produced the highest amount of total anthraquinones, were selected for the induction of S. alata. root cultures.
Establishment of S. alata. root cultures
The roots of selected and nonselected plantlets were used as the explants for establishment of S. alata. root cultures. Preliminary experiment of varying the concentration of NAA in B5 medium supplemented with 1 mg l−1 kinetin was investigated in this study. It was found that increasing amount of NAA caused callus forming in appearance. B5 medium supplemented with 0.5 mg l−1 NAA and 1.0 mg l−1 kinetin was an appropriate medium for induction of the root culture. The green cells at the root tips were observed within 1 week after the initiation of the root explant in B5 liquid medium. The old cells were changed in color to brown cells whereas the root tips were still green. Therefore, the root cultures were maintained in B5 medium supplemented with 0.5 mg l−1 NAA and 1.0 mg l−1 kinetin by subculturing every 4 weeks.
Anthraquinone formation in S. alata. root and cell cultures
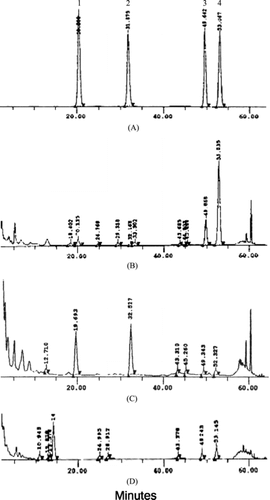
After several subcultures, the root cultures of S. alata. were examined for their potential of anthraquinone production using HPLC. The chromatograms of the extracts obtained from root culture, leaves and root of S. alata. exhibited difference in the chemical patterns. The chromatogram of S. alata. leaf extract exhibited the peaks of aloe-emodin and rhein, while those of the root cultures showed the peaks of chrysophanol, and emodin as the main anthraquinones. The root of the intact plant exhibited the peaks of rhein, chrysophanol, and emodin as the main anthraquinones (). Although peaks with retention times about 20 and 32 min were detected in extracts of the root cultures, they were not identified as aloe-emodin and rhein, respectively, due to different UV absorption spectra. Therefore, the root cultures of S. alata. were capable of producing emodin and chrysophanol but not rhein and aloe-emodin.
Figure 3 HPLC chromatograms of (A) authentics: aloe-emodin (1), rhein (2), emodin (3), and chrysophanol (4); (B) root culture extract; (C) intact leaf extract; and (D) intact root extract.
Although root culture of S. alata. could not produce aloe-emodin and rhein, it produced higher amounts of emodin and chrysophanol than the leaves and roots of the intact plant. Emodin and chrysophanol are the main bioactive components of S. alata.. It has been reported that emodin and chrysophanol have various activities such as anticancer activity and antifungal activity (Agarwal et al., Citation2000; Shieh et al., Citation2004). The ability of the root culture of S. alata. to produce emodin and chrysophanol in higher amounts than the intact plant suggests that it can be the material of choice for biosynthetic studies of emodin and chrysophanol. Such ability implies that various enzymes involved in the biosynthetic pathway of emodin and chrysophanol are operating under these culture conditions.
The root cultures that were established from high- and low-anthraquinone-producing plantlets produced emodin and chrysophanol in significantly different amounts (). The root culture obtained from the high-anthraquinone-yielding plantlets produced emodin and chrysophanol that were 16- and 26-times higher, respectively, than that obtained from the low-yielding plantlets. This result supports the strategy for increasing secondary metabolite production in plant tissue cultures by using high-producing plantlets as initiation explants (Panichayupakaranant, Citation2001).
Table 1. Anthraquinone content in root cultures, leaves and roots of S. alata.
It should also be noted that this gradient high-performance liquid chromatographic method with photodiode arrays detection is highly effective for the simultaneous determination of anthraquinone content, including aloe-emodin, rhein, emodin, and chrysophanol in S. alata. and related plants. The method is fast and does not require prior purification of the sample extracts.
Acknowledgment
The authors wish to thank the Graduate School, Prince of Songkla University, for a research grant.
References
- Agarwal JS, Rastogi RP, Srivastava OP (1976): In vitro. toxicity of constituents of Rumex maritimus. Linn. to ringworm fungi. Curr Sci 45: 619–620. [CSA]
- Agarwal SK, Singh SS, Verma S, Kumar S (2000): Antifungal activity of anthraquinone derivatives from Rheum emodi.. J Ethnopharmacol 72: 43–46. [INFOTRIEVE], [CSA], [CROSSREF]
- Charlwood BV, Rhodes MJC (1990): Secondary Products from Plant Tissue Culture. Oxford, Clarendon Press. pp. 1–18.
- Elujoba AA, Ajulo OO, Iweibo GO (1989): Chemical and biological analyses of Nigerian Cassia. species for laxative activity. J Pharmaceut Biomed 7: 1453–1457. [CSA], [CROSSREF]
- Farnsworth NR, Bunyapraphatsara N (1992): Thai Medicinal Plant, Recommended for Primary Health Care System. Bangkok, Prachachon, pp. 90–93.
- Panichayupakaranant P (2001): Naphthoquinone formation in cell cultures of Impatiens balsamina.. Pharm Biol 39: 293–296. [CSA]
- Shieh D, Chen Y, Yen M, Chiang L, Lin C (2004): Emodin-induced apoptosis through p53-dependent pathway in human hepatoma cells. Life Sci 74: 2279–2290. [INFOTRIEVE], [CSA], [CROSSREF]
- Zhou X, Song B, Jin L, Hu D, Diao C, Xu G, Zou Z, Yang S (2006): Isolation and inhibitory activity against ERK phosphorylation of hydroxyanthraquinones from rhubarb. Bioorg Med Chem Lett 16: 563–568. [INFOTRIEVE], [CSA], [CROSSREF]