Abstract
Four novel chemical constituents, cannabiside B, cannabiside C, cannabiloid B, and cannabilactone A, have been isolated from the n.-butanol extract of the herb of Senecio cannabifolius. Less. (Compositae). The structures of the isolated compounds were established as 3-[3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-5-hydroxyfuran-2(5H)-one (1), 8,10-dihydroxy-7-(hydroxymethyl)-4-methyl-1, 6-dioxaspiro [4.5] dec-3-en-2-one (2), 6-(1,2-dihydroxy-ethyl)-3-[3-(3,4-dihydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-hydroxy-pyrrol-2-ylidene]-4-hydroxy-tetrahydro-pyran-2-one (3), and 5-hydroxy-3-{[5-(hydroxylmethyl)furan-2-yl] methylene} benzofuran-2(3H)-one (4), respectively, by spectroscopic (UV, IR, NMR, and MS) methods. All the compounds showed antibacterial activities against Staphylococcus aureus. and Bacillus subtilis. but not Escherichia coli..
Introduction
The plant Senecio cannabifolius. Less. (Compositae) is distributed mainly in the northeast and in the Hebei Province of China, in Korea, Japan and the far east of the former Soviet Union. It is used as a traditional remedy for treating virus influenza, enteritis, and pneumonia in China (Anon., Citation1985; Anon., Citation1999). Plants belonging to the genus are notable for producing a wide variety of pyrrolizidine alkaloids and furoeromophilane sesquiterpenes. Senecio., the large and very diverse genus, has already been studied extensively for its secondary chemicals (Bohlmann et al., Citation1986). In the literature, there exists limited chemical and biological information on S. cannabifolius.; a new pyrrolizidine alkaloid (Asada et al., Citation1982), and a new lactone (Wu et al., Citation2002) have been reported as isolated from this plant, and carcinogenic activity in ACI rats (Hirono et al., Citation1983) and growth inhibitory effects on the larvae of the tobacco cutworm Spodoptera litura. were found (Lajide et al., Citation1996). In the course of the investigation on the chemical constituents of S. cannabifolius., we now wish to report the isolation and structure elucidation of a total of four new compounds, cannabiside B (1), cannabiside C (2), cannabiloid B (3), and cannabilactone A (4) (). The four compounds were found to possess significant antimicrobial activities against Staphylococcus aureus. and Bacillus subtilis. (Gram-positive bacteria), but they were not active against E. coli. (Gram-negative bacterium).
Materials and Methods
Melting points are uncorr. 1H and 13C NMR, distortionless enhancement by polarization transfer (DEPT), correlation spectroscopy (COSY), heteronuclear multiple quantum coherence (HMQC), and heteronuclear multiple bond coherence (HMBC) spectra were measured using a JNM-LA400 (JEOL Datum Ltd., Tokyo, Japan). Chemical shifts are shown in δ (ppm) with tetramethylsilane (TMS) used as an internal reference. UV spectra were recorded on an UV-VIS recording spectrophotometer (Shimadzu UV-2201, Shimadzu Corporaton, Kyoto, Japan) and IR spectra on a Bruker IFS 55 (Bruker Optics GmpH, Faellanden, Switzerland). High resolution electrospray ionization mass spectrometry (HR-ESI-MS) (positive ion-mode) was obtained with a Finnigan LCQ (Thermo Electron Corporation, USA) using a heated capillary temperature of 180°C, a capillary voltage of 13 V, sheath and auxiliary nitrogen gas velocities of 50 a.u. and 10 a.u. in a total-scanning manner. TLC was carried out on precoated Si gel 60 F254 plates (Merck KGaA, Darmstadt, Germany) and RP-18 F254s plates (Merck). Spots were detected under UV (254 and 365 nm) before and after spraying with 10% H2SO4 ethanol solution, followed by heating the plate at 110°C for 5 min. Column chromatography was carried out on Wakogel C-100 (Wako Pure Chemical Industries, Ltd., Osaka, Japan). The HPLC separation was performed on normal phase high-performance liquid chromatography (NP-HPLC) and reversed phase high-performance liquid chromatography (RP-HPLC), the former consisted of the following parts: a Merck-Hitachi system (L-7100 pumps, L-7400 UV detector, L-7490 RID, L-7300 column heater, L-7200 autosample, and D-7000 interface, Hitachi High-technologies Corporation, Tokyo, Japan) and a AQUASIL SS-4251 (60) column (10 mm × 250 mm); the latter was composed of the following parts: a Shimadzu system (LC-6AD pump, SPD-10Avp, UV detector, RID-10A detector, CTO-10ACvp column heater, SCL-10Avp system controller, and SCL-10Advp autoinjector) and a COSMOSIL 5C18-AR-II Waters column (10 mm × 250 mm, Japan Nacalai Tesque).
Plant material
The plant material was supplied by Jilin Hua Kang Pharm. Co., Ltd. (Dunhua, China) in May 2001 and was identified as the herb of S. cannabifolius. Less. by the researcher Dr. Zhong-Gai Yan, Changchun Research Institute of Traditional Chinese Materia Medica, Jilin Province, China, in July 2001.
Extraction and isolation
The plant material was dipped into water overnight (1:10) and then extracted three-times after the material had been boiled for 2 h. Filtration and rotary evaporation of the resulting extract gave a residue (780 g) that was dissolved in water and partitioned by successive solvent extraction with chloroform, ethyl acetate, and n.-butanol, yielding 9.4, 24.4, and 150.0 g, respectively.
The n.-butanol extract (25 g) was passed through a lowbar ODS column (809.1 mg) with a methanol-water (2:8) mixture. According to the TLC control of the fractions, they were collected into four main fractions. Fractions 1 and 2 were subjected separately to HPLC with a methanol-water-tetrahydrofuran (5:95:1) mixture to furnish 1 (42.7 mg), 2 (15 mg), and 3 (14 mg).
The chloroform extract (1.42 g) was chromatographed over lowbar silica gel column (400 g) eluted by a gradient of hexane, ethyl acetate, and methanol. According to the TLC control of the fractions, they were collected into five main fractions. Fraction 2 was subjected to HPLC with a methanol-water-tetrahydrofuran (40:60:1) mixture to furnish 4 (14.9 mg).
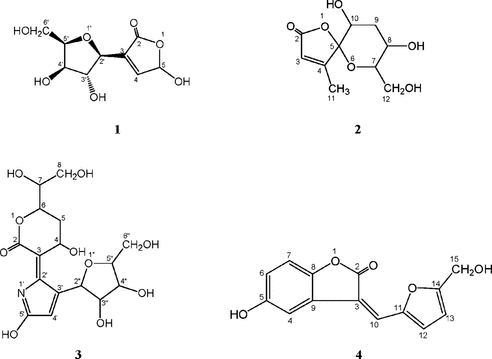
3-[3,4-Dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-5-hydroxyfuran-2(5H)-one (1)
Colorless liquid; [α]D20+130.8° (c. 1.0, MeOH); UV (MeOH) 214, 262nm; IR (1%KBr) νmax 3412, 1693, 1385, 1273, 1025, 998, 824, 765 cm−1; 1H NMR(DMSO-d.6, 400 MHz) δ 7.87 (1H, d, J. = 8.0 Hz, H-4), 5.63 (1H, d, J. = 8.0 Hz, H-5), 11.29 (1H, s, OH-5), 5.76 (1H, d, J. = 5.2 Hz, H-2′), 4.00 (1H, dd, J. = 4.8/9.6 Hz, H-3′), 3.95 (1H, m, H-4′), 3.82 (1H, dd, J. = 3.2/6.8 Hz, H-5′), 3.60 (1H, m, H-6′a), 3.53 (1H, m, H-6′b), 5.37 (1H, d, J. = 4.4 Hz, OH-3′), 5.08 (1H, br.s, OH-4′), 5.08 (1H, br.s, OH-6′); 13C NMR (DMSO-d.6, 400 MHz) δ 163.0 (s, C=O), 150.6 (s, C-3), 140.6 (s, C-4), 101.7 (d, C-5), 87.6 (d, C-2′), 73.5 (d, C-3′), 69.8 (d, C-4′), 84.8 (d, C-5′), 60.8 (t, C-6′); HR-ESI-MS (positive mode) m/z. [M + H]+ 223.0648 (calcd. for C9H13O7, 223.0656).
8,10-Dihydroxy-7-(hydroxymethyl)-4-methyl-1,6-dioxaspiro[4.5]dec-3-en-2-one (2)
Colorless liquid; [α]D20+63.7° (c. 0.13, MeOH); UV (MeOH): 214, 262nm; IR νmax (1% KBr): 3306, 2972, 1703, 1657, 1476, 1433, 1273, 1066 cm−1; 1H NMR (DMSO-d.6, 400 MHz) δ 7.68 (1H, d, J. = 1.2 Hz, H-3), 3.74 (1H, dd, J. = 4.0/6.8 Hz, H-7), 4.22 (1H, m, H-8), 2.07 (1H, m, H-9a), 2.04 (1H, m, H-9b), 6.15 (1H, dt, J. = 1.0/6.4 Hz, H-10), 1.76 (3H, d, J. = 1.2 Hz, H-11), 3.56 (1H, m, H-12a), 3.54 (1H, m, H-12b), 5.23 (1H, d, J. = 6.4 Hz, OH-8), 5.03 (1H, t, J. = 6.4 Hz, OH-12); 13C NMR (DMSO-d.6, 400 MHz) δ 163.6 (s, C=O), 136.0 (d, C-3), 153.0 (s, C-4), 109.3 (s, C-5), 87.2 (d, C-7), 70.4 (d, C-8), 39.4 (t, C-9), 83.6 (d, C-10), 12.3 (q, C-11), 61.3 (t, C-12); HR-ESI-MS (positive mode) m/z. [M + H]+ 231.0872 (calcd. for C10H15O6, 231.0863).
6-(1,2-Dihydroxy-ethyl)-3-[3-(3,4-dihydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-hydroxy-pyrrol-2-ylidene]-4-hydroxy-tetrahydro-pyran-2-one (3)
Colorless liquid; [α]D20+101.2° (c. 0.12, MeOH); UV (MeOH): 210nm; IR νmax (1% KBr): 3422, 2925, 1689, 1606, 1384, 1067 cm−1; 1H NMR (DMSO-d.6, 400 MHz) δ 4.93 (1H, ddd, J. = 7.2/9.2/11.2 Hz, H-4), 2.50 (1H, m, H-5a), 2.13 (1H, q, J. = 11.2 Hz, H-5b), 4.62 (1H, ddd, J. = 4.4/5.6/11.2 Hz, H-6), 3.75 (1H, p, J. = 5.6 Hz, H-7), 3.40 (2H, t, J. = 5.6 Hz, H-8), 6.92 (1H, d, J. = 7.2 Hz, OH-4), 5.21 (1H, d, J. = 5.6 Hz, OH-7), 4.75 (1H, t, J. = 5.6 Hz, OH-8), 7.98 (1H, s, H-4′), 11.69 (1H, s, OH-5′), 5.71 (1H, d, J. = 6.0 Hz, H-2″), 4.43 (1H, q, J. = 6.0 Hz, H-3″), 4.08 (1H, q, J. = 4.0 Hz, H-4″), 3.87 (1H, q, J. = 4.0 Hz, H-5″), 3.58 (1H, m, H-6″a), 3.50 (1H, m, H-6″b), 5.42 (1H, d, J. = 6.0 Hz, OH-3″), 5.18 (1H, d, J. = 4.0 Hz, OH-4″), 4.98 (1H, d, J. = 5.2 Hz, OH-6″); 13C NMR (DMSO-d.6, 400 MHz) δ 174.7 (s, C=O), 152.1 (s, C-3), 51.1 (d, C-4), 29.6 (t, C-5), 77.9 (d, C-6), 71.5 (d, C-7), 62.4 (t, C-8), 156.3 (s, C-2′), 136.9 (s, C-3′), 117.4 (s, C-4′), 150.8 (s, C-5′), 87.0 (d, C-2″), 74.4 (d, C-3″), 70.8 (d, C-4″), 85.6 (d, C-5″), 61.8 (t, C-6″); HR-ESI-MS (positive mode) m/z. [M + H]+ 390.1367 (calcd. for C16H22NO10, 390.1395).
5-Hydroxy-3-{[5-(hydroxylmethyl)furan-2-yl]methylene}benzofuran-2(3H)-one (4)
Yellow needles; [α]D20−24.5° (c. 0.11, MeOH); mp 224∼226°; UV (MeOH): 206, 209, 234, 257, 364nm; IR νmax (1% KBr): 3227 (OH), 1758 (C=O), 1610, 1572, 1460, 1287, 1136, 1079, 1007, 807, 670 cm−1; 1H NMR (DMSO-d.6, 400 MHz) δ 7.82 (1H, d, J. = 2.4 Hz, H-4), 6.79 (1H, dd, J. = 2.4/8.8 Hz, H-6), 7.05 (1H, d, J. = 8.8 Hz, H-7), 7.45 (1H, s, H-10), 7.35 (1H, d, J. = 3.4 Hz, H-12), 6.68 (1H, d, J. = 3.4 Hz, H-13), 4.69 (2H, d, J. = 6.0 Hz, CH2-15), 9.45 (1H, s, OH-5), 5.55 (1H, t, J. = 6.0 Hz, OH-15); 13C NMR (DMSO-d.6, 400 MHz) δ 165.5 (s, C=O), 115.2 (s, C-3), 122.2 (s, C-9), 110.9 (d, C-4), 153.7 (s, C-5), 116.8 (d, C-6), 110.7 (d, C-7), 146.2 (s, C-8), 123.1 (d, C-10), 149.3 (s, C-11), 124.1 (d, C-12), 110.8 (d, C-13), 161.9 (s, C-14), 56.2 (t, C-15); HR-ESI-MS (positive mode) m/z. [M + H]+ 259.0623 (calcd. for C14H11O5, 259.0601).
Antibacterial susceptibility testing
Growth inhibitory activity of these compounds was tested against three bacteria (Staphylococcus aureus. IFO 3060, Bacillus subtilis. IFO 3007, Escherichia coli. HUT 215). Bacterial strains were obtained from the microbiology laboratory of the University of Kochi, Faculty of Agriculture, Japan. Proper amounts of each fraction were obtained to form the solution of the crude drug (100 g/ml equivalent) with 80% ethanol, and it was diluted with sterile distilled water before the experiment. Then we transferred 100 µl of sample solution into the microtube (1.5 ml) and diluted it with 900 µl of distilled water in order to change the concentration to be 10 g/ml eq.
The qualitative antibacterial bioassay of each extract employed was the agar-well diffusion assay. Chloramphenicol at 0.2 mg/l was employed as a positive control. LB liquid medium was prepared by mixing 10 g of peptone, 5 g of yeast, and 5 g of NaCl solution together in 1000 ml distilled water, adjusting pH value to be 7.2 with 2 N NaOH solution. Then, 15 g of agar were added to LB liquid medium to obtain LB agar solid medium. The bacteria were incubated, respectively, in three test tubes with 3 ml of LB liquid medium with shaking at 37°C for 18 h. The inocula of target strains were injected into the LB agar solid medium sterilized at 121°C for 15 min (100 µl of each inoculum). Then the agar medium was dispensed into a sterilized culture dish with seven stainless steel wells (interior diameter, 6 mm; external diameter, 8 mm; height, 10 mm). After the medium was solidified, we removed the stainless steel wells and added 100 µl of sample solution to each well, repeating with every diluted concentration (10 g/ml eq). Finally, the culture dish was sealed. The diameters of the inhibition zones were measured using a hand-held digital caliper after incubation for 24 h at 30°C to yield mean values (two times; ).
Table 1. Antibacterial activities of extracts of S. cannabifolius.
MIC values of purified compounds were determined by turbidimetry. The correct amount of purified compound was dissolved in DMSO to achieve a solution with a concentration of 10 mg/ml. The solution (500 µl) was drawn by a micropipette before transferring to a graduated centrifuge tube, diluted with sterile distilled water (1:1), and mixed with shaking by the mini-oscillator to change the concentration to be 5 mg/ml. Then, another 500 µl of solution were drawn and transferred to the next centrifuge tube before diluting it with 500 µl of distilled water. The operation was repeated. As a result, a series of the sample solutions in decreasing concentration was obtained by a ratio of 0.5 (final concentration: 10 mg/ml to 9.77 µg/ml). A 100 µl aliquot of solution was drawn from each sample of different concentration and the solutions were added into the test tubes that had been prepared by the addition of 900 µl of LB liquid medium. After the inocula of target strains were uniformly added (20 µl), a lid was placed on the tubes followed by incubation at 30°C with shaking for 48 h. Growth was followed by spectrophotometry (Shimadzu UV-1200) at 600 nm using DMSO as control to measure the absorbance. The MIC value was defined as the lowest concentration to inhibit visible growth. DMSO solution (10%) did not interfere with the microbial growth. Bacteria counts were effected using an hemocytometer (Staphylococcus aureus. 1.6 cfu/ml, Bacillus subtilis. 1.3 cfu/ml, Escherichia coli. 2.5 cfu/ml).
Results and Discussion
Compound 1 was obtained as a colorless liquid with the molecular formula of C9H12O7 by the HR-ESI-MS. In the 1H NMR spectrum, correlations were displayed from the alkenyl proton at δ 7.87 (1H, d, J. = 8.0 Hz) to C-5 (δ 101.7), C-3 (δ 150.6), and C-2 (δ 163.0) and from the proton at δ 5.63 (1H, d, J. = 8.0 Hz), which bonded with the carbon (δ 101.7) bearing two oxygen atoms to C-2 (δ 163.0). There were three exchangeable proton resonances at δ 11.29 (1H, s), 5.37 (1H, d, J. = 4.4 Hz) and 5.08 (2H, br.s) characteristic of active hydroxyl functions. Correlations were observed from hydroxyl protons at δ 5.08 (2H, br.s) to C-6′ (δ 60.8), C-3′ (δ 73.5), and C-5′ (δ 84.8), which was also correlated with two methylene protons at δ 3.53 (1H, m) and 3.60 (1H, m) and from OH-3′ to C-4′ (δ 69.8), C-3′ (δ 73.5), and C-2′ (δ 87.6). There was discerned long-range connectivity from H-4 to C-2′ (δ 87.6) in the HMBC spectrum. Correlations were observed from H-2′ to C-4 (δ 140.6) and C-3 (δ 150.6). On the basis of these spectroscopic studies, it was concluded that 1 was 3-[3,4-dihydroxy-5- (hydroxymethyl)tetrahydrofuran-2-yl]-5-hydroxyfuran-2(5H)-one. Because it was reported for the first time, we named it cannabiside B. The relative stereochemistry of 1 was determined using a nuclear overhauser effect spectroscopy (NOESY) experiment, aided by a Dreiding molecular model. NOESY correlations between H-4′/H-5′, and H-5′/H-2′, and the fact that there was no correlation observed between H-4′/H-3′ and H-3′/H-2′ indicating that H-2′, H-4′, and H-5′ lie on the same side of the molecule.
Compound 2 was shown to have a molecular formula of C10H14O6 as a colorless liquid by HR-ESI-MS with four degrees of unsaturation. Correlations were observed from H-3 to C-11 (δ 12.3), C-4 (δ 150.3), and C-2 (δ 163.6) and from H-11 to C-5 (δ 109.3), C-3 (δ 136.0), and C-2 (δ 163.6) in the correlation through long-range coupling (COLOC) spectrum. There were about 3 or 4 carbon signals in the sp.2 hybridized region of the 13C-NMR spectrum and one exchangeable proton resonance at δ 5.03 (1H, t, J. = 6.4 Hz) in the 1H NMR spectrum. In the 1H-1H COSY spectrum, correlations were observed from OH-12 to H-12, from H-12 to H-7, from H-7 to H-8, from H-8 to OH-8 and H-9, and from H-9 to H-10. Thus, the compound 2 was characterized as 8,10-dihydroxy-7-(hydroxymethyl)-4-methyl-1,6-dioxaspiro[4.5]dec-3-en-2-one. It was proved to be a novel chemical, so it was named cannabiside C.
The HR-ESI-MS of compound 3 showed a [M + H+] at m/z. 388 as a colorless liquid, corresponding to the molecular formula C16H21NO10 implying seven elements of unsaturation. There were 6 carbon signals in the sp.2 hybridized region of the 13C NMR spectrum, among which the resonance at δ 174.7 was assigned to carbonyl carbon. The compound was indicated to be glycoside analogue because of a series of O.-bearing and sp.3 hybridized carbon signals at δ 60–100. There were seven exchangeable proton resonances at δ 11.69 (1H, s), 6.92 (1H, d, J. = 7.2 Hz), 5.42 (1H, d, J. = 6.0 Hz), 5.21 (1H, d, J. = 5.6 Hz), 5.18 (1H, d, J. = 4.0 Hz), 4.98 (1H, d, J. = 5.2 Hz), and 4.75 (1H, t, J. = 5.6 Hz) assigned to hydroxyl groups. Correlations were observed from H-8 to C-7 (δ 71.5) and C-6 (δ 77.9); from H-4 to C-3 (δ 152.1), C-2′ (δ 156.3), and C-2 (δ 174.7); from H-2″ to C-3″ (δ 74.4), C-3′ (δ 136.9), and C-5′ (δ 150.8); and from H-3′ to C-4′ (δ 117.4) and C-5′ (δ 150.8) in the HMBC spectrum. In the 1H-1H COSY spectrum, there were also discerned long-range connectivities from OH-7 to H-7, from H-7 to H-6, from H-6 to H-5, from H-5 to H-4, from OH-4 to H-4, from OH-6″ to H-6″, from H-6″ to H-5″, from H-5″ to H-4″, from H-4″ to OH-4″ and H-3″, and from H-3″ to OH-3″ and H-2″. The novel compound was thus assigned the structure 6-(1,2-dihydroxy-ethyl)-3-[3-(3, 4-dihydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-hydroxy-pyrrol-2-ylidene]-4-hydroxy- tetrahydropyran-2-one and named cannabiloid B.
Compound 4 was obtained from the chloroform extract of S. cannabifolius. as yellow needles, which may bear phenolic hydroxyl function indicated by positive FeCl3-K3[Fe(CN)6] reaction. The molecular formula of 4 was determined to be C14H10O5 by the HR-ESI-MS. Fourteen carbon signals could be observed in the 13C NMR spectrum and one sp.3 hybridized methylene group, six sp.2 hybridized methines, and seven sp.2 hybridized quaternary carbons in the DEPT 135° spectrum, among which the resonance at δ 169.5 was assigned to carbonyl group. The proton resonances at δ 7.05 (1H, d, J. = 8.8 Hz), 6.79 (1H, dd, J. = 8.8/2.4 Hz), and 7.82 (1H, d, J. = 2.4 Hz) formed the typical AMX coupling system. There were two exchangeable proton resonances at δ 9.45 (1H, s) and 5.55 (1H, t, J. = 6.0 Hz) characteristic of hydroxyl functions. Long-range connectivities were observed from hydroxyl proton (OH-5) to C-5 (δ 153.7), C-6 (δ 116.8), and C-4 (δ 110.9) implying the hydroxyl moiety was attached between HM and Hx, from HA(δ 7.05) to C-5 (δ 153.7), C-8 (δ 146.2), C-9 (δ 122.2), and C-6 (δ 116.8); from HM (δ 6.81) to C-8 (δ 146.2) and C-7 (δ 110.7); from HX (δ 7.84) to C-5 (δ 153.7), C-8 (δ 146.2), C-6 (δ 116.8), and C-3 (δ 115.2); from OH-15 to C-15 (δ 56.2) and C-14 (δ 161.9); from H-15 to C-13 (δ 110.8), and C-14 (δ 161.9); from H-12 to C-14 (δ 161.9), C-11 (δ 149.3), and C-13 (δ 110.8); from H-13 to C-11 (δ 149.3) and C-12 (δ 124.1); and from H-10 to C-2 (δ 165.5), C-11 (δ 149.3), C-12 (δ 124.1), C-9 (δ 122.2), and C-3 (δ 115.2). According to the IR spectrum and the presence of 10 elements of unsaturation, the compound should have the lactone skeleton. Therefore, compound 4 was identified as 5-hydroxy-3-{[5-(hydroxylmethyl)furan-2-yl] methylene}benzofuran-2(3H)-one. This is the first report of the isolation of compound 4, so we gave the name cannabilactone A to the new compound.
The agar-well diffusion assay was used to screen for antimicrobial activity of the four isolated compounds. The minimum inhibitory concentrations (MICs) detected by the turbidimetry are listed in . They were found to have significant antibacterial activities against both Gram-positive bacteria, but not the Gram-negative bacterium, although their activities were less potent than those of chloramphenicol.
Table 2. Antibacterial activities of compounds 1–4 (MIC values in µg/ml)
Acknowledgment
The authors are grateful to Master Katsuyosi Tanaka, Faculty of Agriculture Kochi University, for providing technical support to this study.
References
- Anon. Editor Committee of National Chinese Medical Manage Bureau “Chinese Herb” (1999): Chinese Herb 7. Shanghai, Shanghai Science and Technology Publisher, p. 943.
- Anon. Plant Research Institute of Academia Sinica (1985): Claves Familiarum Generumque Cormophytorum Sinicorum. Beijing, Science Press, p. 445.
- Asada Y, Furuya T, Shiro M, Nakai H (1982): Studies on constituents of crude drugs. Part XIII. Seneciannabine, a new pyrrolizidine alkaloid from Senecio cannabifolius.. Tetrahedron Lett 23: 189–192. [CSA]
- Bohlmann F, Zdero C, Jakupovic J, Grenz M, Castro V, King RM (1986): Further pyrrolizidine alkaloids and furoeremophilanes from Senecio. species. Phytochemistry 25: 1151–1159. [CSA], [CROSSREF]
- Hirono I, Ueno I, Aiso S, Yamaji T, Haga M (1983): Carcinogenic activity of Farfugium japonicum. and Senecio cannabifolius.. Cancer Lett 20: 191–198. [INFOTRIEVE], [CSA], [CROSSREF]
- Lajide L, Escoubas P, Mizutani J (1996): Cyclohexadienones-insect growth inhibitors from the foliar surface and tissue extracts of Senecio cannabifolius.. Experientia 52: 259–263. [CSA], [CROSSREF]
- Wu B, Wu LJ, Wang LQ (2002): A new lactone from Senecio cannabifolius.. J Asian Nat Prod Res 4: 315–317. [INFOTRIEVE], [CSA], [CROSSREF]