Abstract
The capacity of a chemical to produce liver damage in vivo. often results from interaction of a series of complex cellular processes that are involved in the uptake, biotransformation, and elimination of potentially toxic compounds. Reactive oxygen species (ROS) are involved in the pathophysiology of a number of human ailments including carbon tetrachloride–induced hepatotoxicity. Several phytochemicals found in natural products associated with pharmacological attributes have been reported to scavenge free radicals. Chronic hepatotoxicity was induced by intraperitoneal injection of CCl4 (1.5 ml/kg body wt.) in paraffin oil. Administration of ethyl acetate extract of Phellinus rimosus. (Berk) Pilat (Hymenochaetaceae) 25 and 50 mg/kg body wt. orally prior to CCl4 injection significantly and dose-dependently protected the CCl4-mediated elevation of serum transaminases such as glutamate pyruvate transaminase (GPT) and glutamate oxaloacetate transaminase (GOT), and of serum alkaline phosphatase (ALP). The hepatic antioxidant status, superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities and the reduced glutathione (GSH) level was reduced in the CCl4 alone treated animals. Administration of extract prior to CCl4 challenge restored the hepatic antioxidant status. The antioxidant status in the blood of CCl4 alone treated animals also decreased compared with the normal as well as extract plus CCl4 treated animals. The activity of a phase I enzyme, aniline hydroxylase, showed profound increase in the CCl4 alone treated animals that was reduced in the extract plus CCl4 treated group. The activity of phase II enzyme glutathione S.-transferase (GST) was also elevated in the liver and blood of animals treated with the extract compared with the CCl4 alone treated group. The serum and liver lipid peroxidation levels were elevated in the CCl4-treated animals compared with the normal or extract plus CCl4 treated animals. The findings thus suggested ethyl acetate extract of P. rimosus. protects CCl4-induced chronic hepatotoxicity in rat by restoring the liver antioxidant status, inhibiting phase I and enhancing phase II enzyme activities.
Keywords:
Introduction
During the past decade, considerable attention has been focused on the involvement of oxygen free radical (OFR) in various diseases (Hemnani & Parihar, 1998). Antioxidants have a wide range of biochemical activities, including inhibition of generation of reactive oxygen species directly or indirectly, scavenging free radicals, and altering intracellular redox potential (Hirose et al., Citation1994). Despite the presence of strong antioxidant defense mechanism to counteract the OFR and to minimize plausible oxidative damage, OFR-dependent damage to DNA and other biomolecules accumulates during the lifetime of organisms. The liver protects the body from potentially injurious substances (endotoxins) absorbed from the intestinal tract, as well as the toxic by-products of metabolism. The most important in the detoxification process is that of the microsomal drug metabolizing system of the liver. A large number of xenobiotics are reported as potentially hepatotoxic. Among these agents, predictable reactions are ascribed to acetaminophen, tetracycline, antineoplastic agents, ethanol, and carbon tetrachloride. Hepatotoxins may react with the basic cellular constituents (proteins, lipids, RNA and DNA) and induce almost all types of lesions of the liver (Guillouzo, Citation1998).
Most of the chemicals must undergo metabolic activation by phase I enzymes to form electrophilic reactants, which can interact with nucleophilic groups in macromolecules including DNA. It has been demonstrated that active oxygen molecules, such as superoxide and hydroxyl radicals, play an important role in the inflammation process after intoxication by ethanol, carbon tetrachloride, or carrageenan (Yoshikawa et al., Citation1983; Halliwell & Gutteridge, Citation1984; Yuda et al., Citation1991). The incidence of hepatic disease mirrors the lifetime incidence of hepatocellular carcinomas (HCC) in humans in high-risk areas of China, Southeast Asia, and Africa (Benson et al., Citation1979; Zhu et al., Citation1988). Hence the search for liver protective agents more amenable for use in man led to the evaluation of nontoxic hepatoprotective natural products. Protection has been achieved by administering chemopreventive agents that modulate the metabolic processing of xenobiotics including phenolic antioxidants, indoles, isothiocynates, coumarins, flavonones, allylsulfides, and so forth. (Kensler, Citation1997). Carbon tetrachloride (CCl4) is a constituent of many dry-cleaning fluids, and its main toxic effect is found on the liver, although there is some injury to other tissues. Administration of antioxidants such as vitamin E, promethazine, propyl gallate, and reduced glutathione (GSH) or of the compound SKS-525A, which inhibits microsomal drug metabolism, decreases CCl4-induced toxicity in animals (Halliwell & Gutteridge, Citation1985a).
Mushrooms are macrofungi, and they have had a notable place in folk medicine throughout the world since ancient times. Attempts have been made in many parts of the world to explore the use of mushrooms and their metabolites for the treatment of a variety of human suffering (Jong & Birmingham, Citation1992). Phellinus. species (Hymenochaetaceae) are polypore macrofungi, mostly tropical, and 18 species are known from Kerala, India. One of the species, P. lintus. (Berk. et Curt.) Teng is reported to possess in vitro. antioxidant, anti-angiogenic, and xanthine oxidase inhibiting activities (Song et al., Citation2003). Phellinus rimosus. (Berk) Pilat is often found growing on jackfruit tree trunks in Kerala (India). Some local tribes, for the treatment of mumps, have used the basidiocarps of this fungus. Our earlier investigations showed that ethyl acetate and methanol extracts of P. rimosus. possessed antioxidant, anti-inflammatory, hepatoprotective, and nephroprotective activities (Ajith & Janardhanan Citation2001Citation2002; Ajith et al., Citation2002). Our earlier investigations on the hepatoprotection of P. rimosus. against CCl4-induced acute toxicity in rats prompted us to extend our investigation to CCl4-induced chronic hepatotoxicity in order to elucidate its possible antioxidant-mediated hepatoprotection and the effect on phase I and phase II enzyme activities.
Materials and Methods
Animals
Female Sprague-Dawley rats (10-weeks-old, 160–180 g) purchased from the Small Animal Breeding Center, Kerala Agriculture University (Mannuthy, Kerala, India), were used for the studies. They were maintained in environmentally controlled conditions with free access to standard food (Sai Durga Feeds, Bangalore, India) and water. The rats were acclimatized for 1 week before initiating the experiment. The experiments were conducted according to the permission and prescribed guidelines of the institutional animal ethics committee.
Chemicals
Carbon tetrachloride (CCl4), aniline, phenol, hydrogen peroxide (H2O2), and chloroform (CHCl3) were purchased from Merck India Ltd (Mumbai, India). Nitroblue tetrazolium (NBT), reduced glutathione (GSH), reduced nicotinamide adeninedinucleotide tetrasodium (NADH), 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB), 1-chloro-2,4-dinitrobenzene (CDNB), and riboflavin were from Sisco Research Laboratories Ltd (Mumbai, India). All other chemicals and reagents used were of analytical grade.
Preparation of the extract
Sporocarps of P. rimosus. were collected from the outskirts of Thrissur, India. The specimen was identified with available literature, and the identification was confirmed by Professor K.M. Leelavathi, Department of Botony Calicut University (Calicut, India). A voucher specimen was deposited in the herbarium of the Centre for Advanced Studies in Botany, University of Madras (Chennai, India) (HERB. MUBL. 3171). The sporocarps were cut into small pieces, dried at 40–50°C for 48 h, and powdered. Two hundred grams of the powder was defatted by extraction with petroleum ether. The defatted material was then extracted for 8–10 h with ethyl acetate using a Soxhlet apparatus (Ajith & Janardhanan, Citation2002). The extract was completely evaporated at 40°C using a vacuum evaporator. The residue, designated as ethyl acetate extract (2.5 g), was employed for the experiments. The extract was dissolved in minimum volume of ethanol and diluted with distilled water to form a uniform suspension. The ethanol remaining in the solution was allowed to evaporate at 40–45°C.
Determination of hepatoprotective effect
Animals were divided into four groups of six animals each. Group I, treated with vehicle (distilled water), was kept as normal. Group II, injected with CCl4 in paraffin oil (1:5, v/v) (1.5 ml/kg body wt., i.p) 3-times in a week for 5 weeks (total 15 doses), was kept as control. Groups III and IV were treated orally with the ethyl acetate extract of P. rimosus. 25 and 50 mg/kg body wt. 1 h before each CCl4 administration. The animals were sacrificed 24 h after the last injection of CCl4. Coagulated and noncoagulated (heparin) blood were collected directly from the heart of each animal. Serum was used for the determination of glutamate pyruvate transaminase (GPT) (Reitman & Frankel, Citation1957), glutamate oxaloacetate transaminase (GOT) (Reitman & Frankel, Citation1957), and alkaline phosphatase (ALP) (Kind & King, Citation1954). Serum lipid peroxidation (malondialdehyde; MDA) was estimated (Ohkawa et al., Citation1979) after precipitating the total protein by the method of Satoh (Citation1987). 1,1,3,3-Tetramethoxypropane was used as the standard for MDA. Liver was removed for the determination of antioxidants and also for histopathological observations.
Evaluation of antioxidants in liver
Liver was removed and washed thoroughly in ice-cold saline, and a homogenate (10%) was prepared in PBS (50 mM, pH 7). A part of the homogenate was used for the estimation of reduced glutathione (GSH) (Moron et al., Citation1979). The remaining homogenate was centrifuged at 5000 × g. for 10 min in a centrifuge at 4°C, and after removal of the cell debris, the supernatant was used for the assay of superoxide dismutase (SOD) (Mc Cord & Fridovich, Citation1969), catalase (CAT) (Beers & Sizer, Citation1952), glutathione peroxidase (GPx) (Hafemann, et al. Citation1974), aniline hydroxylase (Bourrie et al., Citation1996), lipid peroxidation (Ohkawa et al., Citation1979), and glutathione S.-transferase (GST) (Habig et al., Citation1974). Protein was determined by the method of Lowry et al. (Citation1951).
Evaluation of antioxidants in blood
Noncoagulated (heparin) blood was used for the determination of antioxidant status. Blood was centrifuged at 2000 × g. for 5 min and plasma separated. SOD activity was measured in erythrocyte lysate prepared after removing the hemoglobin according to the method of Miami and Yoshikawa (Citation1979). CAT was estimated by the method of Aebi (Citation1974), selenium dependent GPx estimated by the method of Hafmann et al. (Citation1974), a 20% lysate of blood in distilled water was used for the estimation of GSH according to the method of Moron et al. (Citation1979), and GST was estimated in erythrocyte lysate (Habig et al., Citation1974). Hemoglobin was determined using Drabkin's reagent (Drabkin & Austin, Citation1932).
Histopathological examination
A portion of the liver was fixed in 10% formalin and then embedded in paraffin. Microtome sections of 6 µm thickness were prepared from each liver and stained with hematoxylin-eosin. The sections were evaluated for the pathological assessment of hepatotoxicity such as necrosis, fatty infiltration, fibrosis, lymphocyte infiltration, and so forth.
Statistical analysis
All data are represented as mean±SD. Experimental data were statistically analyzed using one-way analysis of variance (ANOVA) by MSTAT-C software package. The control group (CCl4 alone treated group) and the extract plus CCl4 treated groups were further analyzed by Dunnett's t.-test. P values less than 0.05 were considered as significant.
Results
Hepatoprotection
Chronic exposure to CCl4 significantly (p < 0.05) elevated the serum GOT and GPT activities (). The SGPT and SGOT activities in the CCl4 alone injected animal were 959.8±25.6 and 231.1±21.5 U/l, respectively. The activity was significantly lowered by the ethyl acetate extract of P. rimosus. in a dose-dependent manner. The inhibition of activity of the serum transaminases (SGPT and SGOT) were 73.4% and 66.1%, respectively, compared with the control group animals. Similarly, the serum ALP activity was elevated significantly (p < 0.05) in the CCl4-treated control animals. The inhibition of ALP activity by the extract was 52.8% with respect to the control animals. The serum MDA level was also elevated in the CCl4 alone injected group. The level was reduced significantly in the extract-treated group.
Table 1. Effect of ethyl acetate extract (EtOAc) of P. rimosuson. serum GOT, GPT and ALP activities in rats with chronic CCl4 administration
Antioxidant status in liver
The activities of SOD, CAT, GPx, GST, and GSH were decreased significantly (p < 0.05) in the CCl4 alone treated group. The activity of SOD in the CCl4 injected animals was 14.5 ± 2.1 U/mg protein. Treatment of extract (50 mg/kg body wt.) prior to the CCl4 challenge enhanced the activity to 19.1 ± 0.8 U/mg protein (). The activities of CAT and GPx in the CCl4 alone treated animals were 46.3 ± 4.8 U/mg protein and 16.9 ± 0.8 U/mg protein, respectively (). The activity was restored to normal in the extract plus CCl4 treated animals.
Table 2. Effect of ethyl acetate extract (EtOAc) of P. rimosus. on hepatic SOD, CAT, and GPx activities in rats with chronic CCl4 administration
The level of GSH was also elevated to that of the normal animal in the extract-treated animals prior to the CCl4 challenge (). The level was 7.94 ± 0.65 nmol/mg protein and 11.38 ± 0.43 nmol/mg protein in the CCl4 alone and extract (50 mg/kg body wt.) plus CCl4 treated animals, respectively.
Table 3. Effect of ethyl acetate extract (EtOAc) of P. rimosus. on blood and hepatic GSH level in rats with chronic CCl4 administration
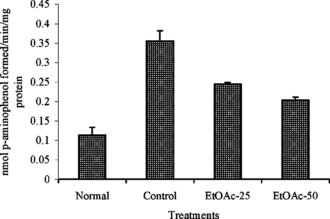
The activity of aniline hydroxylase was significantly (p < 0.05) elevated in the CCl4 alone treated group (). Administration of extract before the CCl4 injection inhibited the activation of aniline hydroxylase enzyme. The activity in the CCl4 and extract plus CCl4 was 0.356 ± 0.020 and 0.204 ± 0.007 nmol of p.-aminophenol formed min−1 mg protein−1, respectively.
Figure 1 Effect of ethyl acetate extract (EtOAc) of P. rimosus. on hepatic aniline hydroxylase activity in rats treated with chronic doses of CCl4. Values are mean ± SD, n = 2.
The activity of hepatic GST was elevated in the extract plus CCl4 treated animals, 1149.4 ± 44.0 and 1407.2 ± 89.1µmol of CDNB conjugate formed min−1 mg protein−1 for 25 and 50 mg/kg body wt. treated group (). The activity in the CCl4 alone treated animals was 775.6 ± 105.7 µmol of CDNB conjugate formed min−1 mg protein−1.
Table 4. Effect of ethyl acetate extract (EtOAc) of P. rimosus. on blood and hepatic GST level in rats with chronic CCl4 administration
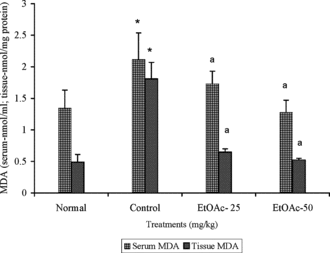
The level of MDA was 2.10 ± 0.04 nmol/ml and 1.85 ± 0.26 nmol/mg protein in the serum and liver of CCl4 alone treated animals (). The extract (50 mg/kg body wt.) treatment prior to CCl4 challenge reduced the serum and liver MDA level to 1.20 ± 0.20 and 0.53 ± 0.02 nmol/mg protein, respectively.
Figure 2 Effect of ethyl acetate extract (EtOAc) of P. rimosus. on serum and MDA level in rats with chronic CCl4 administration. Values are mean ± SD, (n = 6 in serum and n = 5 in tissue). *p < 0.05 (LSD) significantly different from normal. ap < 0.01 (Dunnett's t.-test) significantly different from control group.
Antioxidant status in blood
The activities of erythrocyte SOD, CAT, GPx, GST, and blood GSH were observed to be lowered in the CCl4 alone injected group. The activities were restored to normal in animals treated with extract before CCl4 treatment. The SOD activity in the CCl4 plus extract (50 mg/kg body wt.) treated group was 1481.6 ± 63.2 U/g Hb (). Similarly, the activity of CAT in the CCl4 alone injected animals was 66.8 ± 5.7 k/g Hb and in the extract (50 mg/kg body wt.) plus CCl4 treated animals was 95.6 ± 3.6 k/g Hb.
Table 5. Effect of ethyl acetate extract (EtOAc) of P. rimosuson. erythrocyte SOD, CAT, and GPx activities in rats with chronic CCl4 administration
The treatment of extract prior to the CCl4 challenge enhanced the glutathione antioxidant system such as GPx, GST, and GSH. The activity of GPx (), GST (), and GSH () in the extract (50 mg/kg body wt.) plus CCl4 treated animals was 5102.4 ± 803.1 U/g Hb, 20.10 ± 1.51 mmol of CDNB conjugate formed min−1 g Hb−1 and 3.87 ± 0.09 µmol/ml,respectively.
Histopathological observations
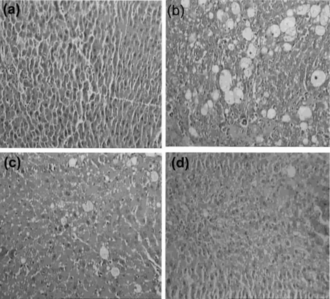
Histopathological observations showed severe necrosis, fatty infiltration, fibrosis, and lymphocytes infiltration in the hepatocyte of CCl4 alone treated animals (). The effects were moderate to low in the liver of extract (25 and 50 mg/kg body wt.) treated animals.
Figure 3 Hepatoprotective activity of ethyl acetate extract (EtOAc) of P. rimosus. against CCl4-induced chronic hepatotoxicity in rats. Liver sections stained with H&E. (a) Normal; (b) CCl4/paraffin oil; (c) EtOAc (25 mg/kg body wt.) + CCl4;; (d) EtOAc (50 mg/kg body wt.) + CCl4. Magnification, × 20.
Discussion
Results of the current study reveal the significant and dose-dependent hepatoprotective activity of the ethyl acetate extract of P. rimosus. against chronic hepatotoxicity induced by CCl4. The activity of transaminases (SGPT and SGOT) was elevated significantly in the serum of CCl4 alone injected animals. The elevation of SGPT in the serum indicates the necrosis of the hepatocyte and, hence, the altered ratio of SGPT to SGOT in animals injected with CCl4. The elevated serum ALP activity was due to the intrahepatic cholestasis, which was reduced significantly in the extract-treated animals. Pretreatment of the extract prevented the elevation of SGPT, SGOT, and serum ALP activities consequent to CCl4 injection, indicating the hepatoprotective activity of the extract.
The antioxidant status of the hepatocytes was altered in the CCl4 alone treated animals. The treatment of ethyl acetate extract of P. rimosus. prior to the CCl4 injection effectively protected the decline of antioxidant activity. CCl4 is metabolized in Cyt P450 system to give the trichloromethyl radical (CCl3•). Trichloromethyl radical reacts with oxygen to form trichloroperoxy radical (CCl3O2•); both these products induce the peroxidation of lipids (Ahr et al., Citation1982). The products of peroxidation are known to inhibit protein synthesis and activity of certain enzymes. Administration of CCl4 alone decreased the activity of CAT and GPx. Further, the activity of SOD and the level of GSH were also reduced in the liver. The declined antioxidant enzyme activity is responsible for the increased lipid peroxidation, which leads to loss of membrane fluidity, membrane integrity, and finally loss of cell functions of liver (Smith, et al., Citation1987; Halliwell & Gutteridge, Citation1989). This may result in the leakage of enzymes, toxic metabolites, and free radicals to circulation. The treatment of the extract prior to the CCl4 injection increased the hepatocyte SOD, CAT, and GPx activities and could effectively prevent radical-mediated loss of membrane integrity. Hence, the extract-treated animals showed reduced transaminase activity in the serum.
The role of GSH in the formation of conjugates with electophilic drug metabolites most often formed by cytochrome P450 linked monooxygenase is well established (Mitchell et al., Citation1973). Studies with a number of models show that the hepatotoxicity of xenobiotics often is produced by GSH depletion (Jollow et al., Citation1974; Rana & Tyal, Citation1981). The decreased concentration of GSH increases the sensitivity to oxidative and chemical injury. Exogenous GSH could offer protection against CCl4-induced injury in rats (Rana & Tyal, Citation1981). The results of the current study also support these findings. The treatment of extract before the CCl4 injection prevented the decline of hepatic GSH level. GSH protects hepatocyte by forming the substrate for the GPx and reacts directly with intermediates produced during the peroxidation of membrane lipid.
Treatment of animals with extract plus CCl4 enhances the activity of Se-GPx (selenium-dependent GPx) compared with the CCl4 alone treated animals. The enhanced GPx activity could partially explain the protection of biomembrane from oxidative attack. The protective role of Se-GPx against CCl4-induced hepatotoxicity has been reported in the rat (Rana & Kumar, Citation1993). The fat accumulation in the liver of CCl4-treated animals is due to blockage in the synthesis of lipoproteins that carry triacylglycerol away from the liver. Histopathological observation of the CCl4 and CCl4 plus extract treated liver clearly shows the level of fatty infiltration and necrosis due to radical-mediated cytotoxicity.
Because liver is very active in GSH biosynthesis and is translocated to blood, significant reduction of GSH levels in extrahepatic tissue and blood indicates hepatic damage (Rana et al., Citation2002). This is evident from the decreased level of GSH in the blood of animals treated with CCl4 alone. The level was enhanced moderately in the extract plus CCl4 treated animals. The reduced activity of SOD, CAT, and Se-GPx in the erythrocytes of CCl4 alone treated animals indicated the fragility of erythrocytes to the toxic metabolites and radicals released from liver. Pretreatment with extract before the CCl4 challenge protects the liver, reducing the release of toxic metabolites and radicals that may normalize the antioxidant enzyme activity in the erythrocytes.
The decline in the antioxidant status of both liver and blood partially explains enhanced MDA in the serum of CCl4 alone treated animals. The level of serum MDA was lowered in the extract plus CCl4 treated animals in a dose-dependent manner. This is due to enhanced antioxidant status in the liver as well as in the blood of extract plus CCl4-treated animals. Erythrocytes contain high concentrations of polyunsaturated fatty acids (PUFA), ferrous ions, and molecular oxygen, which makes them highly vulnerable to oxidative stress (Yadav et al., Citation1997). Studies indicated erythrocytes from selenium-deficient animals are more susceptible to hemolysis in vitro. under conditions favoring lipid peroxidation, relative to normal erythrocytes (Halliwell & Gutteridge, Citation1985b).
The amount of ultimate toxic substance available for interaction with its target represents, in part, a balance between competing activating and detoxifying reactions (Conney, Citation1982). Although this balance is under genetic control, it is easily modulated by a variety of factors including exposure to drugs and other xenobiotics (Conney, Citation1982). Compounds that significantly elevate activation of phase II enzyme without activation of phase I enzyme would be more desirable candidates to render protection from chemically induced liver toxicity. Direct measurement of phase II enzyme activities in blood cells have also been used to assess enzyme induction. Total GST activity, a phase II enzyme, in the hepatocyte is enhanced significantly in the extract plus CCl4-treated animals, relative to normal or control animals. This enhanced GST activity in the erythrocyte may reflect its possible induction by the extract in the liver. Treatment with antioxidant showed elevation of α rather than the μ or π class subunits of liver GST (Kensler, Citation1997). Such increased activity of GST protects liver against damage by electrophilic metabolites. Neverthless, enhanced level of the hepatic GSH, a substrate of GST, could facilitate conjugation of glutathione to the active electrophilic radicals of CCl4 and reduce the hepatotoxicity in the extract plus CCl4 treated group.
Elevation of aniline hydroxylase, a phase 1 enzyme, in the liver of CCl4 alone treated animals and the inhibition of its activity by the extract support the hepatoprotective effect. The enhanced activity of phase 1 enzyme in the CCl4 alone treated animals indicated the increased metabolism of CCl4 and toxicity of liver to active metabolites of CCl4. While inhibiting the activation of phase I enzyme, ethyl acetate extract of P. rimosus. renders protection against CCl4-derived toxic metabolites.
The doses of P. rimosus. were selected based on the previous studies in which we had reported the in vitro. radical-scavenging activity of the ethyl acetate extract of P. rimosus. (Ajith & Janardhanan, Citation2002). Prelimnary phytochemical analysis of the ethyl acetate extract showed the presence of polyphenols and flavonoids (Sheena et al., Citation2003). These components in the extract were responsible for the exhibited property. The direct radical-scavenging activity of the extract might also be involved in the hepatoprotective activity against chronic CCl4 exposure. The results of the current study indicate that the hepatoprotective effect of ethyl acetate extract of P. rimosus. is mediated through the antioxidant defense mechanism.
Acknowledgments
The financial support of the Council of Scientific Industrial Research (CSIR), New Delhi, is gratefully acknowledged. The valuable help of Dr. Ramadasan Kuttan, Director, Amala Cancer Research Centre, Kerala, India, during this study is also gratefully acknowledged.
References
- Aebi H (1974): Catalse. In: Bergmeyer HU, ed., Methods in Enzymatic Analysis. New York, Academic Press, pp. 673–684.
- Ahr HJ, King LJ, Nastainczyk W, Ullrich V (1982): The mechanism of reductive dehalogenation of halomethane by liver cytochrome P450. Biochem Pharmacol 31: 383–387. [INFOTRIEVE], [CSA]
- Ajith TA, Janardhanan KK (2001): Antioxidant and anti-inflammatory activities of methanol extract of Phellinus rimosus.. Ind J Exp Biol 39: 1166–1169. [CSA]
- Ajith TA, Janardhanan KK (2002): Antioxidant and antihepatotoxic activities of Phellinus rimosus. (Berk) Pilat. J Ethnopharmacol 81: 387–391. [INFOTRIEVE], [CSA], [CROSSREF]
- Ajith TA, Jose N, Janardhanan KK (2002): Amelioration of cisplatin induced nephrotoxicity in mice by ethyl acetate extract of a polypore fungus, Phellinus rimosus.. J Exp Clin Cancer Res 21: 487–491. [CSA]
- Beers RF, Sizer IW (1952): A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195: 133–140. [INFOTRIEVE], [CSA]
- Benson AM, Cha YN, Bueding E, Heine HS, Talalay P (1979): Elevation of extrahepatic glutathione S.-transferases and epoxide hyrdase activities by 2(3)-tert.-butyl-4-hydroxyanisole. Cancer Res 39: 2971–2977. [INFOTRIEVE], [CSA]
- Bourrie M, Meunier V, Berger Y, Fabre G (1996): Cytochrome P450 isoform inhibitors as a tool for the investigation of metabolic reactions catalyzed by human liver microsomes. J Pharmacol Exp Therapeutics 227: 321–332. [CSA]
- Conney AH (1982): Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: GHA Clowers Memorial Lecture. Cancer Res 42: 4875–4917. [INFOTRIEVE], [CSA]
- Drabkin DL, Austin JM (1932): Spectrophotometric studies, spectrometric constants for common haemoglobin derivatives in human, dog and rabbit blood. J Biol Chem 98: 719–733. [CSA]
- Guillouzo A (1998): Liver cell models in in vitro. toxicology. Environ Health Perspect 106: 511–532. [INFOTRIEVE], [CSA]
- Habig WH, Pabst MJ, Jakoby WR (1974): Glutathione S.-transferase. The first enzymatic step in mercapturic acid formation. J Biol Chem 249: 7130–7139. [INFOTRIEVE], [CSA]
- Hafemann DG, Sunde RA, Houestra WG (1974): Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutrition 104: 580–584. [CSA]
- Halliwell B, Gutteridge JMC (1984): Lipid peroxidation, oxygen radicals; cell damage and antioxidant therapy. Lancet 1: 1396–1397. [INFOTRIEVE], [CSA], [CROSSREF]
- Halliwell B, Gutteridge JMC (1985a): Free radicals and toxicology. In: Halliwell B, Gutteridge JM, eds., Free Radicals in Biology and Medicine. Oxford, Clarendon Press, pp. 206–243.
- Halliwell B, Gutteridge JMC (1985b): Lipid peroxidation a radical chain reaction. In: Halliwell B, Gutteridge JM, eds., Free Radicals in Biology and Medicine. Oxford, Clarendon Press, pp. 139–189.
- Halliwell B, Gutteridge JMC (1989): Free radicals, aging and disease. In: Halliwell, B, Gutteridge JMC, eds., Free Radicals in Biology and Medicine. Oxford, Clarendon Press, pp. 416–425.
- Hemnani T, Gutteridge JMC (1998): Reactive oxygen species and oxidative DNA damage. Indian J Physiol Pharmacol 42: 440–452. [INFOTRIEVE], [CSA]
- Hirose M, Imaida K, Tamano S, Ito N (1994): Cancer chemoprevention by antioxidants. In: Ho CT, Oswa T, Huang MT, Rosen RT, eds., Food Phytochemicals II: Teas, Spices and Herbs. Washington, DC, American Chemical Society, pp. 122–132.
- Jollow DJ, Mitchell JR, Zampaglione N, Gillete JR (1974): Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11: 151–156. [INFOTRIEVE], [CSA]
- Jong SC, Birmingham JM (1992): Edible mushrooms in biotechnology. Proc Asian Mycol Symp. Seoul, Korea. 18–35.
- Kensler TW (1997): Chemoprevention by inducers of carcinogen detoxification enzymes. Environ Health Perspect 105: 965–970. [INFOTRIEVE], [CSA]
- Kind PRN, King EJ (1954): Estimation of plasma phosphatase by determination of hydrolyzed phenol with antipyrene. J Clin Pathol 7: 322–326. [INFOTRIEVE], [CSA]
- Lowry HD, Rosenberg NJ, Farr AL, Randa RJ (1951): Protein measurement with Folin phenol reagent. J Biol Chem 193: 265–275. [INFOTRIEVE], [CSA]
- Mc Cord JM, Fridovich I (1969): Superoxide dismutase, an enzymatic function for erythrocuprein. J Biol Chem 244: 6049–6055. [CSA]
- Miami M, Yoshikawa H (1979): A simplified assay method of superoxide dismutase activity for clinical use. Clin Chim Acta 92: 337–342. [CSA], [CROSSREF]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB (1973): Acetaminophen induced hepatic necrosis. Protective role of glutathione. J Pharmacol Exp Ther 187: 211–215. [INFOTRIEVE], [CSA]
- Moron MA, Depierre JW, Mannervik B (1979): Levels of glutathione reductase and glutathione S-transferase. activities in rat lung and liver. Biochem Biophys Acta 582: 67–78. [INFOTRIEVE], [CSA]
- Ohkawa H, Ohishi N, Yagi K (1979): Assay for lipid peroxide in animal tissues by thiobarbituric acid reaction. Ann Biochem 95: 351–358. [CSA], [CROSSREF]
- Rana S, Tyal MK (1981): Influence of zinc, vit-B12 and glutathione on the liver of rats exposed to carbon tetrachloride. Industrial Health (Japan) 19: 65–69. [CSA]
- Rana SVS, Kumar S (1993): Antioxidant enzymes in the liver of rat treated with carbon tetrachloride after parathyroidectomy. Physiol Chem Phys Med 25: 41–46. [CSA]
- Rana SVS, Allen T, Singh R (2002): Inevitable glutathione, then and now. Indian J Exp Biol 40: 706–716. [INFOTRIEVE], [CSA]
- Reitman S, Frankel AS (1957): A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28: 56–63. [INFOTRIEVE], [CSA]
- Satoh K (1987): Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta 90: 37–43. [CSA]
- Sheena N, Ajith TA, Mathew T, Janardhanan KK (2003): Antibacterial activity of three macrofungi, Ganoderma lucidum, Navesporus floccosa. and Phellinus rimosus. occurring in South India. Pharm Biol 41: 564–567. [CSA]
- Smith SM, Grishsm MB, Nancy EA, Granger DA, Kvietys PR (1987): Gastric mucosal injury in the rat. Role of iron and xanthine oxidase. Gastroeneterology 92: 950–956. [CSA]
- Song YS, Kim SH, Sa JH, Jin C, Lim CJ, Park EH (2003). Anti-angiogenic, antioxidant and xanthine oxidase inhibition activities of the mushroom Phellinus lintus.. J Ethnopharmacol 88: 113–116. [INFOTRIEVE], [CSA], [CROSSREF]
- Yadav P, Sarkar S, Bhatnagar D (1997): Lipid peroxidation and antioxidant enzymes in erythrocytes and tissue in aged diabetic rats. Indian J Exp Biol 35: 389–392. [INFOTRIEVE], [CSA]
- Yoshikawa T, Tanaka H, Yoshida N, Seto O, Sugino N, Kondo M (1983): Adjuvant arthritis and lipid peroxide protection by superoxide dismutase. Lipid Peroxide Res 7: 108–110. [CSA]
- Yuda Y, Tanaka J, Hirano F, Igarani K, Satch T (1991): Participation of lipid peroxidation in rat pertussis vaccine pleurisy. Chem Pharm Bull 39: 505–506. [INFOTRIEVE], [CSA]
- Zhu YR, Chen JG, Huang XY (1988): Hepatocellular carcinoma in Qidong Country. In: Tang ZY, Wu NC, Xia SS, eds., Primary Liver Cancer. Beijing, China, Academic Publishers, pp. 204–222.