Abstract
This paper describes comparative in vitro. and in vivo. anthelmintic activities of Vernonia anthelmintica. (L.) Willd. (Compositae) seeds and levamisole. In vitro. studies revealed higher anthelmintic effects (p ≥ 0.05) of crude methanol extract (CME) as compared with crude aqueous extract (CAE) of V. anthelmintica. seeds on live Haemonchus. contortus. as evident from their mortality. For in vivo. studies, seeds of V. anthelmintica. were administered as crude powder (CP), CAE, and CME to sheep naturally infected with mixed species of gastrointestinal nematodes. In vivo., maximum reduction (73.9%) in fecal egg counts per gram (EPG) was recorded in sheep treated with V. anthelmintica. CAE at 3 g kg−1 body weight on day 5 post-treatment (PT) followed by CP at 3 g kg−1 (55.6%) on day 3 PT. However, CME did not exhibit anthelmintic activity in vivo.. It was found that whereas V. anthelmintica. seeds possess anthelmintic activity against nematodes, it was not comparable to levamisole (97.8% to 100% reduction in EPG). It may be suggested that further research on a large scale be carried out with a large number of animals on higher doses than those used in the current study; for the identification of active principles; and for standardization of the doses and toxicity studies for drug development.
Introduction
Helminthiasis is among the most important animal diseases inflicting heavy production losses. The disease is highly prevalent particularly in third-world countries (Dhar et al., Citation1982). Chemical control of helminths coupled with improved management has been the important worm control strategy throughout the world. However, the worldwide increase in resistance of gastrointestinal trichostrongylids of domestic small ruminants against conventional anthelmintics (Prichard, Citation1990; Hertzberg & Bauer, Citation2000) and the resulting economical damage demonstrate the urgent need for alternative methods to reduce the worm burden in the animal. Plants have been used from ancient times to cure diseases of man and animals. This system of therapy is commonly referred as Unani, folk, Eastern, or indigenous medicine in Indo-Pakistan (Nadkarni, Citation1954). There are some reports on plants with anthelmintic properties from Asia and Africa (Akhtar et al., Citation2000; Alawa et al., Citation2003; Lateef et al., Citation2003; Iqbal et al., Citation2004), America (Pessoa et al., Citation2002), and Europe (Waller et al., Citation2001) that have shown that plants can be a good alternative for the treatment of helminthosis.
Several Vernonia. species are used widely in native cultures as folklore remedies in different parts of the world (Johri & Singh, Citation1997). V. anthelmintica. (L.) Wild. (Compositae), locally named kaliziri. in Pakistan, is widely used in the traditional medicine system of Indo-Pakistan subcontinent in mixed prescriptions as a remedy for different ailments both in humans and animals. To mention a few, the plant is used for malaria fever, worms, pain, inflammation, infections, diuresis, cancer, abortion, and various gastrointestinal disorders (Nadkarni, Citation1954; Chopra et al., Citation1956; Hsu, Citation1967; Jain & Puri, Citation1984; Singh & Ali, Citation1989; Bhattarai, Citation1991; Bajpai et al., Citation1995; Grainger, Citation1996). V. anthelmintica. has been used empirically as a dewormer in Ayruveda (Nadkarni, Citation1954; Chopra et al., Citation1956; Said, Citation1969). Scientific evidence of anthelmintic activity of V. anthelmintica. has, however, been reported in combination with other plants; for example, V. anthelmintica., Butea monospermsa. and Carica papaya. against oxyurids of mice (Mehta & Parashra, Citation1966), and V. anthelmintica. and Embelia. ribes. as crude powder (CP) and crude methanol extract (CME) against gastrointestinal nematodes of goats (Javed & Akhtar, Citation1990). Hördegen et al. (Citation2003), however, reported ineffectiveness of V. anthelmintica. plus E. ribes. as alcohol extract in experimental haemonchosis in sheep.
The current study was carried out to evaluate the anthelmintic activity of V. anthelmintica. seeds against mixed gastrointestinal nematode infection in sheep.
Materials and Methods
Plant material
Vernonia anthelmintica. seeds were procured from a local market (Faisalabad, Punjab), and were identified and authenticated by a botanist by comparison with specimens stored in the herbarium of the Department of Botany, University of Agriculture (Faisalabad, Pakistan). The voucher specimen (no. 04/2001; Vernonia anthelmintica. seeds) is stored in the Ethnoveterinary Research and Development Centre (EVRDC), Faculty of Veterinary Science, University of Agriculture (Faisalabad, Pakistan). The seeds were dried in shade, ground fine with an electric grinder, and stored in cellophane bags at 4°C until used.
Preparation of aqueous extract
The crude aqueous extract (CAE) of the ground seeds of V. anthelmintica. was prepared according to the standard method (Fenado et al., Citation1989). Briefly, 100 g of the powdered flowers was mixed with 500 mL of distilled water in a 1-L flask and boiled for 1.5 h. After cooling to 40°C, the “brew” was filtered using Whatman no. 1 filter paper. The filtrate was then concentrated in a vacuum rotary evaporator and the extract (yield: 9.8% w/w) stored at 4°C until required.
Preparation of methanol extract
Powdered V. anthelmintica. seeds were exhaustively extracted with methanol in a Soxhlet apparatus (Asuzu & Onu, Citation1994). The CME (yield: 11.5% w/w) was evaporated to dryness and stored at 4°C until used.
In vitro. anthelmintic activity
The in vitro. trials for anthelmintic activity of CAE and CME were conducted on mature live Haemonchus contortus. of sheep as described previously (Sharma et al., Citation1971). Briefly, the female mature worms were collected from the abomasums of freshly slaughtered sheep in the local abattoir. The worms were washed and finally suspended in phosphate-buffered saline (PBS). Ten worms were exposed in triplicate to each of the following treatments in separate Petri dishes at room temperature (25–30°C):
levamisole, 0.55 mg mL−1;
CAE of V. anthelmintica. seeds at 25 mg mL−1;
CME of V. anthelmintica. seeds at 25 mg mL−1;
PBS (control).
The inhibition of motility and/or mortality of the worms subjected to the above treatments were used as the criteria for anthelmintic activity. The motility was recorded after 0, 1, 2, 3, and 6 h intervals. Finally, the treated worms were kept for 30 min in the lukewarm fresh PBS to observe the revival of motility.
In vivo. anthelmintic activity
The in vivo. trials were conducted at Livestock Experiment Station, Rakh Kherewala (Punjab, Pakistan). A total of 40 Thalli sheep of both sexes aged 7–9 months, weighing 17–22 kg were randomly selected for in vivo. trials. Before the start of the experiment, the animals were confirmed to be naturally infected with mixed species of gastrointestinal nematodes by fecal examination using standard qualitative and quantitative parasitological procedures (Soulsby, Citation1982). Identification of nematode eggs in the feces was done using standard description of MAFF (Citation1979) and Thienpont et al. (Citation1979). The animals selected were suffering from mixed gastrointestinal nematodes species including mainly H. contortus., Trichostrongylus. colubriformis., T. axei., Strongyloides papillosus., and Trichuris ovis.. The experimental sheep (n = 40) were randomly divided into eight groups of five animals each and assigned different oral (p.o) treatments as single dose as given below:
Group 1: untreated control.
Group 2: levamisole HCl (Nilverm 1.5%, w/v; ICI Pakistan Limited, Animal Health Division, Karachi, Pakistan) at 7.5 mg kg−1 body weight (b.w.).
Group 3: CP at 1 g kg−1 b.w.
Group 4: CP at 3 g kg−1 b.w.
Group 5: CAE at the equivalent dose rate 1 g kg−1 b.w. of CP.
Group 6: CAE at the equivalent dose rate 3 g kg−1 b.w. of CP.
Group 7: CME at the equivalent dose rate 1 g kg−1 b.w. of CP.
Group 8: CME at the equivalent dose rate 3 g kg−1 b.w. of CP.
Fecal samples of each group were collected in the morning, starting from day 0 pretreatment and at days 3, 5, 10, and 15 post-treatment (PT) and evaluated for the presence of worm eggs by salt floatation technique (MAFF, Citation1979). The eggs were counted by the McMaster method (Soulsby, Citation1982). Egg count percent reduction (ECR) (Pankavich et al., Citation1973) was calculated using the following formula:
The observations were statistically analyzed using SAS software (SAS, Citation1998). Test of significance between the mean parameters was performed by analysis of variance.
Results and Discussion
In vitro. results indicated the moderate anthelmintic activity of CME of V. anthelmintica. seeds as compared with CAE, but much lower than that of levamisole (). H. contortus. has proved to be a good test worm in in vitro. study because of its longer survival in PBS. By virtue of its longer survival, a greater number of observations was recorded on the motility of worms. This worm and some other strongylides have previously been used for in vitro. studies by some workers (Sharma et al., Citation1971; Prakash et al., Citation1980; Asuzu & Njok, Citation1996; Amorium et al., Citation1998; Sangwan & Sangwan, Citation1998).
Table 1. In vitro. effect of crude aqueous and methanol extracts of Vernonia anthelmintica. on Haemonchus contortus. of sheep in comparison with positive control (levamisole).
In vivo., maximum ECR (73.9%) was recorded in sheep treated with V. anthelmintica. seed CAE at 3 g kg−1 b.w. on day 5 PT followed by CP (ECR = 55.6%) at 3 g kg−1 b.w. on day 3 PT (). The in vivo. anthelmintic activity of CAE was consistent with the in vitro. findings, whereas in vivo. results did not support in vitro. findings in case of CME. The CME did not exhibit anthelmintic activity in vivo. (data not shown). This difference in CME activity may be due to different in vivo. physiological conditions that could have altered the chemistry of active principles.
Figure 1 Reduction in eggs per gram (EPG) of feces in sheep treated with different doses and forms of Vernonia anthelmintica. compared with control group.
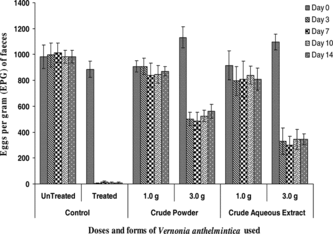
This is the first scientific evidence of anthelmintic activity of V. anthelmintica.. Previously, however, mixed prescriptions of V. anthelmintica. and B.. monosperma. or E. ribes. have been shown to possess anthelmintic effects in mice and goats (Mehta & Parashra, Citation1966; Javed & Akhtar, Citation1990). The prescription (V. anthelmintica. and E. ribes.) as alcohol extracts was reported to be ineffective in experimental haemonchosis in sheep (Hördegen et al., Citation2003).
Therefore, in principle, a comparison cannot be made between the anthelmintic activities of V. anthelmintica. alone (as reported in this study) and mixed prescriptions as described above. The rationale for using mixed prescriptions for anthelmintic purposes in traditional medicine is not available in the literature. However, the synergistic or antagonistic activities of the active principles of the mixed prescriptions of different plants needs to be elaborated.
In the current study, an increase in the dose of both CP and CAE resulted in an increase in the anthelmintic activity (). The higher doses also resulted in early onset of activity. Graded dose response substantiated the presence of bioactive compound(s) responsible for anthelmintic activity in both CP and CAE of seeds of V. anthelmintica.. A minor fluctuation in EPG was, however, observed among various days post-treatment, which was lesser in case of CAE as compared with that in animals treated with CP.
The fluctuation in EPG can be attributed to the variety of nematode species, differences in their fecundity and susceptibility to the drug, and environmental influences. The varying susceptibility of different species of nematodes is substantiated by Hördegen et al. (Citation2003) who found no anthelmintic activity of V. anthelmintica. and E. ribes. mixture against H. contortus. while Trichostrongylus colubriformis. number decreased after this treatment. Therefore, the assumptions made above would be logical, particularly when seen with an almost similar fluctuation in EPG in untreated group of animals (data not shown).
Some components of the seed, such as vernodlin, vernodalol, and vernolic acid, have been isolated and identified (Asaka et al., Citation1977; Lambertini, et al., Citation2004) and are known to have bitter taste and these bitter principles may be responsible for the anthelmintic activity of V. anthelmintica.. Other extracts from Vernonia. species, such as the n.-hexane from the leaves of V. brasiliana. L. Druce, showed an in vitro. antiplasmodial activity, which was attributed to lupeol (Alves et al., Citation1997). Sesquiterpene lactones such as vernolepin, vernolin, vernolide, vernodalin, and hydroxyvernodalin, isolated from V. amygdalina. leaves, have been reported to exhibit antiplasmodial activity against Plasmodium falciparum. strains (Phillipson et al., Citation1993). In the current study, the active principle(s) responsible for the anthelmintic activity seems to be water soluble. This can, to some extent, be supported by more persistent anthelmintic effect and lesser fluctuation in EPG in animals treated with CAE as compared with CP (). The anthelmintic activity of V. anthelmintica. may be attributed to its sesquiterpene lactones vernodalin, vernolide, and hydroxyvernolide, and steroid-related constituents vernonioside BI and vernoniod BI (Gasquet et al., Citation1985; Koshimizu et al., Citation1994). Additionally, the anthelmintic property of V. anthelmintica. may be due to a cathartic effect (Al Magboul et al., Citation1997; Awe & Makinde, Citation1999) as reported in different Vernonia. species, but further studies are needed to confirm all these speculations.
Conclusions
Based on the results of the current study, the use of V. anthelmintica. seeds as an anthelmintic in the form of water decoction is recommended. Quality controlled extracts of V. anthelmintica. seeds alone or possibly isolated bioactive compounds could be a promising alternative to conventional anthelmintics for the treatment of gastrointestinal trichostrongylides of small ruminants in the future. Such a treatment could be used in control strategies against gastrointestinal nematodes in organic and conventional production systems. For this purpose, controlled experiments for the toxicity and efficacy of different extracts and fractions of V. anthelmintica. at different doses against different species of nematodes are recommended for further studies.
Acknowledgment
This research was funded by the University of Agriculture, Faisalabad, Pakistan, under the promotion of research program.
References
- Akhtar MS, Iqbal, Z, Khan MN, Lateef M (2000): Anthelmintic activity of medicinal plants with particular reference to their use in animals in the Indo-Pakistan subcontinent. Small Rumin Res 38: 99–107. [CSA]
- Alawa CBI, Adamu AM, Gefu JO, Ajanusi OJ, Abdu PA, Chiezey NP, Alawa JN, Bowman DD (2003): In vitro. screening of two Nigerian medicinal plants (Vernonia amygdalina. and Annona senegalensis.) for anthelmintic activity. Vet Parasitol 113: 73–81. [INFOTRIEVE], [CSA], [CROSSREF]
- Al Magboul AZI, Bashir AK, Khalid SA, Farouk A (1997): Antimicrobial activity of vernolepin and vernodalin. Fitoterapia 68: 83–84. [CSA]
- Alves TMD, Nagem TJ, de Carvallo LH, Krefft AU, Zani CL (1997): Antiplasmodial triterpenes from Vernonia brasiliana.. Planta Med 63: 554–555. [INFOTRIEVE], [CSA]
- Amorium AD, Borba HR, Rodrigues MDLDA, Anjos DHDS, Correa DVA (1998): In vitro. effect of aqueous extracts of Chenopodium ambrosioides. L. on first and third larval stages of Strongylidae from horses. Rev Brasil Med Vet 20: 14–16. [CSA]
- Asaka Y, Kubota T, Kulkarni AB (1977): Studies on a better principle from Vernonia anthelmintica.. Phytochemistry 16: 1838. [CSA], [CROSSREF]
- Asuzu IU, Njoku CJ (1996): The anthelmintic effect of Alstonia boonei. bark and Nauclea latifolia. leaf aqueous extracts on Trichostrongylus infective larvae. Fitoterapia 67: 220–222. [CSA]
- Asuzu IU, Onu OU (1994): Anthelmintic activity of the ethanol extract of Piliostigma thonningii. bark in Ascaridia galli. infected chickens. Fitoterapia 4: 291–297. [CSA]
- Awe SO, Makinde JM (1999): Cathartic effect of the leaf extract of Vernonia amygdalina.. Fitoterapia 70: 161–165. [CSA], [CROSSREF]
- Bajpai A, Ojha JK, Sant HR (1995): Medicobotany of the Varanasi district. Int J Pharmacog 33: 172–176. [CSA]
- Bhattarai NK (1991): Folk herbal medicines of Makawanpur district, Nepal. Int J Pharmacog 29: 284–295. [CSA]
- Chopra RN, Nayyar SL, Chopra IC (1956): Glossary of Indian Medicinal Plants. New Delhi, India, Council of Scientific and Industrial Research.
- Dhar DN, Sharma RL, Bansal GC (1982): Gastrointestinal nematodes in sheep in Kashmir. Vet Parasitol 11: 271–277. [INFOTRIEVE], [CSA], [CROSSREF]
- Fernando MR, Wickramasinghe SMDN, Thabrew MI, Karunanayaka EH (1989): A preliminary investigation of the possible hypoglycaemic activity of astercanthus longifolia. J Ethnopharmacol 27: 7–14. [INFOTRIEVE], [CSA], [CROSSREF]
- Gasquet M, Bamba D, Babadjamian A, Balansard G, David PT, Metzger J (1985): Amoebicidal and anthelmintic action of vernolid and hydroxyvernolid isolated from the leaves of Vernonia colorata.. Eur J Med Chem Therap 20: 111–115. [CSA]
- Grainger CR (1996): Medicinal plants of seychelles. J Royal Soc Health 116: 107–109. [CSA]
- Hertzberg H, Bauer C (2000): Anthelminthika-Resistenzen bei Magen-Darm-Strongyliden von Schafen und Ziegen: Aktuelles über Verbreitung, Epidemiologie, Vorbeugemassnahmen und Alternativen zum Anthelminthika-Einsatz. Berl Münch Tierärztl Wschr 113: 122–128. [CSA]
- Hördegen P, Hertzberg H, Heilmann J, Langhans W, Maurer V (2003): The anthelmintic efficacy of five plant products against gastrointestinal trichostrongylids in artificially infected lambs. Vet Parasitol 117: 51–60. [CSA], [CROSSREF]
- Hsu YT (1967): Study on the Chinese drugs used as cancer remedy. J S East Asian Res 3: 63. [CSA]
- Iqbal Z, Lateef M, Ashraf M, Jabbar A (2004): Anthelmintic activity of Artemisia brevifolia. in sheep. J Ethnopharmacol 93: 265–268. [INFOTRIEVE], [CSA], [CROSSREF]
- Jain SP, Puri HS (1984): Ethnomedicinal plants of Jaunsar-Bawar hills (Uttar Pradesh) India. J Ethnopharmacol 12: 213–222. [INFOTRIEVE], [CSA], [CROSSREF]
- Javed I, Akhtar MS (1990): Screening of Vernonia anthelmintica. seeds and Embelia ribes. fruit mixed in equal parts against gastrointestinal nematodes. Pak J Pharm Sci 3: 69–74. [INFOTRIEVE], [CSA]
- Johri RK, Singh C (1997): Medicinal uses of Vernonia species. J Med Arom Plant Sci 19: 744–752. [CSA]
- Koshimizu K, Ohigashi H, Huffman MA (1994): Use of Vernonia amygdalina. by wild chimpanzee: Possible roles of its bitter and related constituents. Physiol Behav 56: 1209–1216. [INFOTRIEVE], [CSA], [CROSSREF]
- Lambertini B, Piva R, Khan MTH, Lampronti I, Bianti N, Borgatti M, Gambari R (2004): Effects of extracts from Bangladeshi medicinal plants on in vitro proliferation of human breast cancer cell lines and expression of estrogen receptor alpha gene. Int J Oncol 24: 419. [INFOTRIEVE], [CSA]
- Lateef M, Iqbal Z, Khan MN, Akhtar MS, Jabbar A (2003): Anthelmintic activity of Adhatoda vesica. roots. Int J Agric Biol 5: 86–90. [CSA]
- MAFF (1979): Parasitological Laboratory Techniques.. Technical Bulletin No. 18. Ministry of Agriculture Fisheries and Food. London, Her Majestey's Stationary Office.
- Mehta RK, Parashra GC (1966): Effect of Butea frondosa., Vernonia anthelmintica. and Carica papya. against oxyurids in mice. Ind Vet J 43: 744–748. [CSA]
- Nadkarni AK (1954): Indian Materia Medica. Bombay, India, Popular Prakashan.
- Pankavich JA, Poeschel GP, Shor AL, Gallo A (1973): Evaluation of levamisole against experimental infections of Ascaridia., Heterakis., and Capillaria. spp. in chickens. Am J Vet Res 34: 501–505. [INFOTRIEVE], [CSA]
- Pessoa LM, Morais SM, Bevilaqua CML, Luciano JHS (2002): Anthelmintic activity of essential oil of Ocimum gratissimum. Linn. and eugenol against Haemonchus contortus.. Vet Parasitol 109: 59–63. [INFOTRIEVE], [CSA], [CROSSREF]
- Phillipson JD, Wright CW, Kirby GC, Warhurst DC (1993): Phytochemistry of some plants used in traditional medicine for the treatment of protozoal diseases. In: Van Beck TA, Bretelar H, eds., International Symposium of the Phytochemical Society of Europe, Abstract Book. Lausanne, Switzerland, University of Lausanne, p. 3.
- Prakash V, Singhal KC, Gupta RR, (1980): Anthelmintic activity of Punica granatum. and Artemisia silversiana.. Indian J Pharmacol 12: 62. [CSA]
- Prichard RK (1990): Anthelmintic resistance in nematodes: Extent, recent understanding and future directions for control and research. Int J Parasitol 20: 515–523. [INFOTRIEVE], [CSA], [CROSSREF]
- Said M (1969): Hamdard Pharmacopea of Eastern Medicine. Karachi, Pakistan, Hamdard National Foundation.
- Sangwan N, Sangwan AK (1998): In vitro. effects of leaf extracts of Melia azedarach. on mortality of Haemonchus contortus.. Ind J Anim Res 32: 70–72. [CSA]
- SAS (1998): Statistical Analysis System: User's Guide. Cary, North Carolina, Statistical Institute.
- Sharma LD, Bahga HS, Srivastava PS (1971): In vitro. anthelmintic screening of indigenous medicinal plants against Haemonchus contortus. (Rudolphi, 1803) of sheep and goats. Indian J Anim Res 5: 33–38. [CSA]
- Singh VK, Ali ZA (1989): Folk medicines of Aligarh (Uttar Pradesh) Indian. Fitoterapia 60: 483–490. [CSA]
- Soulsby EJL (1982): Helminths, Arthropods and Protozoa of Domesticated Animals. London, English Language Book Society.
- Thienpont D, Rochette F, Vanparjis OFJ (1979): Diagnosing Helminthiasis Through Coprological Examination. Beerse, Belgium, Janssen Research Foundation.
- Waller PJ, Bernes G, Thamsborg SM, Sukur A, Richter SH, Ingebrigtsen K, Höglund J (2001): Plants as de-worming agents of livestock in the Nordic countries: Historical perspective, popular beliefs and prospects for the future. Acta Vet Scand 42: 31–44. [INFOTRIEVE], [CSA]