Abstract
Roots of Osbeckia chinensis. L. (Melastomaceae), used by the local community of the North Eastern Region of India, were evaluated for hypoglycemic and anti-hyperglycemic activity. Traditionally, a decoction of the roots is used as folk remedy for a variety of ailments, including diabetes mellitus. The effect of aqueous-methanol (1:4) root extracts of O. chinensis. in reducing blood glucose level at different doses varied with the dosage used in both normal and alloxan-induced diabetic mice. The effects were observed to reach maximum 4 h after administration in normal mice and 6 h in diabetic mice, indicating hypoglycemic and anti-hyperglycemic activities. Dosage of 350 mg/kg body weight and above proved to be toxic to normal mice. In diabetic mice, a pronounced anti-hyperglycemic activity of the extract was observed with no apparent toxicity at the dose of 250 mg/kg body weight. Glucose tolerance in normal and diabetic mice was similarly improved on administration of the extract. Glibenclamide, metformin, and insulin were used as reference drugs.
Introduction
Plant infusions and decoctions have been used as popular medicine in several underdeveloped and developing countries as an alternative treatment for various pathophysiological conditions including diabetes mellitus. The local communities residing in the biodiversity-rich areas of the North Eastern Region of India have traditionally used and relied on herbs for treating various ailments (Kayang et al., Citation2005). This practice has continued even today where the low cost and availability, coupled with the poorly equipped government health facility and rising cost of drugs, has left the rural community with hardly any options but to rely on traditional health care practices. These increasing trends in the use of plants as medicines locally and globally necessitate scientific investigations especially where information regarding toxicity is lacking on such plants or their extracts.
Diabetes mellitus is characterized by hyperglycemia together with biochemical alterations of glucose and lipid metabolism (Davis & Granner, Citation1996). The non-insulin dependent diabetes mellitus (NIDDM) condition, which has assumed epidemic proportion today (Kilberstis & Roberts, 2002), is characterized by reduced circulating concentration of insulin, poor insulin sensitivity or insulin resistant, poor glucose tolerance resulting in high sugar in plasma (Arky, Citation1982). Further, prolonged hyperglycemia leads to other microvascular and macrovascular complications (Steppan et al., Citation2001). Current drugs used for the treatment of diabetes include a few groups of chemical compounds such as the drug biguanide (metformin), which although anti hyperglycemic, does not effect hypoglycemia in the normal subjects (Bailey, Citation1992) and involves extrapancreatic mechanisms (Kessler et al., Citation1975; Karam, Citation1982). Then, there are the sulfonylureas (glibenclamide, tolbutamide, etc.), which are insulin secretagogues, and lately, the new class of drugs known as thiazolidinedions (rosiglitazone, pioglitazone), which enhances target tissue sensitivity to insulin (O'Moore-Sullivan & Prins, Citation2002). From the above, it is apparent that different mechanisms are involved in bringing down blood sugar level in normal or hyperglycemic conditions. However, some, such as biguanides, reportedly have undesirable side effects, and hence there is need for effective, safe, and better oral hypoglycemic agents (Murthy, Citation1995).
Many plants have been reported to exhibit hypoglycemic and/or anti-hyperglycemic activity with or without toxic effects (Bailey & Day, Citation1989; Marles & Farnsworth, Citation1995; Shapiro & Gong, Citation2002). An additional advantage with some of these plants is that they have a hypocholesterolemic effect (Murthy, Citation1995). The effects of plants vis-à-vis diabetes are generally compared with the above-mentioned drugs. Research on medicinal plants per se. could provide useful leads to the development of new chemical alternatives for the treatment of diabetes.
We have earlier reported that roots of Potentilla fulgens. L. (Rosaceae) possess hypoglycemic and anti hyperglycemic properties in mice (Syiem et al., Citation2002). This paper describes the study of Osbeckia chinensis. L. (Melastomaceae), a common plant of the North Eastern Region of India, found at higher altitudes (1500–2000 m mean sea level (MSL)). It is widely used by indigenous tribes of Manipur and Meghalaya for controlling hyperglycemia. Literature survey conducted has shown that there is no report on the hypoglycemic and anti-hyperglycemic effect of O. chinensis. on blood glucose level although a few chemical compounds, like tannins, were isolated (Jend-De Su et al., 1988; Zeng et al., Citation1991).
Materials and Methods
Chemicals
Alloxan was procured from Sigma chemical Co. (St. Louis, MO, USA), glibenclamide was obtained from Hoechst, Insulin from Knoll Pharmaceutical Ltd., metformin from USV limited (Maharashtra, Mumbai, India), while other chemicals used were of analytical grade obtained from E. Merck and Hi-media (Baroda, India).
Test animals
Healthy, adult female Swiss albino mice of approximately the same age group and weighing 20–30 g were used for the study. Mice were housed in a room kept under controlled conditions with temperature maintained at 22°C on a 12-h light/dark cycle and were fed with balanced mice feed obtained from Amrut Laboratory (Pune, India).
Plant material
O. chinensis. was collected during the month of November 2003 from NEHU campus, Shillong, Meghalaya, India. The specimens were submitted and identified (voucher no. 71 NEHU) by herbarium curator Dr. P.B. Gurung Department of Botany, NEHU, Shillong, Meghalaya.
Extraction
Roots of O. chinensis. were separated, weighed, washed, shredded, and dried. The roots (350 g) were then powdered, homogenized, and repeatedly extracted with 10 volumes (3.5 L) of aqueous-methanol solution (1:4) (Harborne, Citation1998). Following the method used earlier (Syiem et al., Citation2002), the mixture was filtered and the filtrate was evaporated to dryness at 40°C. The dried mass obtained was used for the investigation. The yield of methanol extract (w/w from dried starting material) was 6.6%. Prior to use, weighed powder was dissolved in 2% ethanol and centrifuged at low rpm for 10 min. The clear supernatant was used for further study.
Normoglycemic studies
Experimental design
Following the approach used by Rao et al. (Citation2000), mice were divided into four test and one control group to study the effects of varying dose of the extracts of O. chinensis. in normal and diabetic mice. Each group comprised 6 mice (n = 6). Varying doses of the extracts of O. chinensis. ranging from 150 to 450 mg/kg body weight (b.w.) were administered to the test group intraperitoneally (i.p.) and glucose level was monitored at different time intervals up to 24 h after administration of the extract. The control group received only 2% ethanol, being the solvent used for preparation. Food, but not water, was withheld during test period not exceeding 24 h. Food, fluid intake, and body weights were monitored for 4 weeks after administration of the extract.
Anti-hyperglycemic studies
Induction of non-insulin dependent diabetes mellitus (NIDDM)
Animals were administered alloxan monohydrate (150 mg/kg b.w., i.p.) prepared in acetate buffer (0.15 M, pH 4.5) as described earlier (Syiem et al., Citation2002). The control group received only the buffer. Prior to administration, mice were fasted overnight but given water ad libitum.. Mice with more than a three- to fourfold increase in their blood sugar levels were considered diabetic and used for further tests.
Administration of extract to alloxan-induced diabetic mice
Following the same experimental design as with normoglycemic studies, alloxan-induced diabetic mice were administered the test extracts (i.p.) at varying doses (150–450 mg/kg b.w.) and the blood glucose levels were measured at varying time intervals. All animals treated were observed for behavioral changes like polydipsia, polyphagia, and polyurea.
Oral glucose tolerance test (OGTT)
Experimental design
Mice were divided into a control and five test groups to study the glucose tolerance in normal and alloxan-induced diabetic mice after the administration of extracts of O. chinensis. at the optimum dose (250 mg/kg b.w.) determined by ED50, while the reference drugs metformin (Zang & Tan, Citation2000), glibenclamide (Shirwaikar et al., Citation2004), and insulin (Srinivas et al., Citation2000) were administered following the respective cited methods. Each group comprised 6 mice. Normal or alloxan-diabetic mice, fasted overnight but provided water ad libitum., were administered the test samples intraperitoneally 1.5 h prior to the oral glucose load of 2 g/kg b.w., according to the method used earlier (Syiem et al., Citation2002). Glucose concentration was measured before administration and subsequently at 30, 60, 120, 480, and 1440 min after the glucose load. A control group received only the glucose load.
Toxicity studies
Normoglycemic mice were administered up to a dose of 450 mg/kg b.w. and kept under observation for any signs of distress, convulsion, coma, or death (Ghosh, Citation1984).
Collection of blood and determination of blood glucose level
Blood samples from the control and experimental mice were collected by orbital sinus puncture using heparinized capillary glass tubes (Ivorra et al., Citation1988). The blood samples so collected were analyzed for glucose levels employing Glucostix with the glucometer (Ames).
Statistical analysis
Student's t.-test was used for determining the levels of significance between the control and the test values. Results are expressed as mean±SEM.
Results
Normal mice
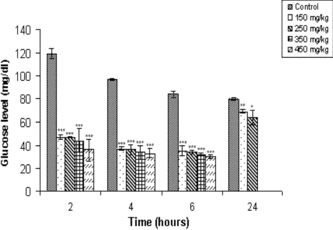
The hypoglycemic effect of O. chinensis. at different doses (150–450 mg/kg b.w.) on normal mice was observed to be dose- and time-dependent. Hypoglycemic effect was observed with the O. chinensis. extract for all the doses used, at 2-, 4-, and 6-h intervals where blood glucose level was reduced by more than 50% from that of control. Higher doses of extracts (350–450 mg/kg b.w.) resulted in marked reduction in blood glucose level, followed by pronounced hypoglycemia and death within 24 h of extract administration (). Optimal reduction was observed at 250 mg/kg b.w., where the blood glucose level was reduced by 63% (p < 0.001) in the fourth hour with no contraindications. Notably, extracts of O. chinensis. administered at the optimal dose exerted prolonged hypoglycemic effects observed even at 24 h with no contraindications.
Alloxan-induced diabetic mice
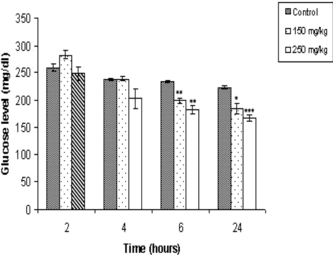
The magnitude of anti-hyperglycemic effects of O. chinensis. extracts varied with respect to the duration of effects and the doses used (). O. chinensis. extracts, at a dosage of 150 mg/kg b.w., reduced blood glucose level by 14% 6 h after extract administration, whereas at 250 mg/kg b.w. the reduction was 21% at 6 h and 25% at 24 h. Significantly, no fatality was observed at these doses ().
Oral glucose tolerance test
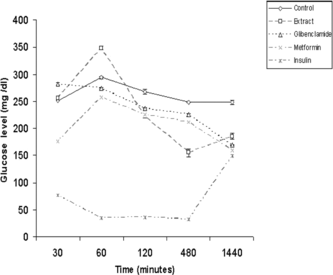
Administration of the extracts (250 mg/kg b.w.) improved glucose tolerance in normal mice (). The effect of O. chinensis. is comparable with the standard oral anti-hyperglycemic drugs metformin and glibenclamide in terms of magnitude of effects and response time. The magnitude of reduction is comparable at the 120- and 480-min time intervals with blood glucose levels reduced by 45% and 58%, respectively. However, while the effects of the standard drugs normalize at 24 h, hypoglycemia was observed to persist for O. chinensis. extracts at 24 h.
Figure 3 Glucose tolerance test in normal mice treated with extracts of O. chinensis. (250 mg/kg) and reference drugs, assayed at different time intervals. Values are expressed as mean±SEM.
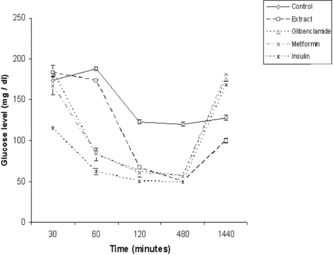
Administration of extracts 1.5 h prior to the glucose load (250 mg/kg b.w.) improved glucose tolerance in diabetic mice. The effects were comparable at 120 min between the extracts and the reference drugs glibenclamide and metformin, wherein blood glucose level reduction was observed to be 17%, 11%, and 10%, respectively. The effect was significantly more effective than either of the standard oral drugs metformin or glibenclamide at 480 min (). Whereas metformin and glibenclamide cause a reduction in the blood glucose level by less than 15% in this time period, the effect of O. chinensis. extracts was observed to reduce blood glucose level by more than 37%.
Discussion
From the study, it is evident that O. chinensis. extract can reduce blood glucose levels in normal as well as alloxan-induced diabetic mice. The magnitude of reduction in blood glucose level in normal and diabetic mice was found to be time- and dose-dependent. The extract exhibited optimal hypoglycemic effect in normal mice at the dose of 250 mg/kg b.w. with no contraindications. O. chinensis. extract also exerted a more rapid hypoglycemic effect even at a lower dose of 150 mg/kg b.w., reducing blood glucose level by 60%, 2 h after extract administration. The hypoglycemic effects were comparable even at the higher doses. Death in normal mice observed at higher doses (350 mg/kg b.w.) is most probably due to pronounced hypoglycemia. However, we cannot rule out other toxic effects at this juncture. The pattern was different in alloxan-induced diabetic mice where β.-cells are partly destroyed. While an antidiabetic effect was observed after extract administration to diabetic mice, notably, the duration of effects was significantly more prolonged, persisting even at 24 h. The more prolonged effect of the extract in diabetic mice may be due to the limited or compromised action of insulin in diabetic condition and, therefore, a greater role for the hypoglycemic principle present in the extract. This effect is different from the plant extracts such as Helicteres isora. L. (Sterculiaceae) (Venkatash et al., Citation2004) and Pterocarpus santalinus. L. (Fabaceae) (Rao et al., Citation2000), which, although anti-hyperglycemic, have no effect in normal mice. The prolonged anti-hyperglycemic effects observed in our study may involve an extrapancreatic mechanism.
The tolerance test in normal mice treated with the extracts and standard reference drugs glibenclamide, metformin, and insulin were compared. The OGTT reflects the extent of intestinal glucose absorption and hepatic glucose metabolism. The suppression of the peak () by the test extracts in normal mice suggested that the extracts contain principle(s) that are comparable with that of glibenclamide and/or metformin but contrasted with insulin. Metformin is known to decrease glucose absorption in the intestine and increase glucose absorption in the liver (Kessler et al., Citation1975; Wilcock & Bailey, Citation1990). Glibenclamide, a sulfonylurea derivative, on the other hand, is a well-known insulin secretagogue, causing hypoglycemia by stimulating pancreatic β-cells to release more insulin, inhibiting glucagon secretion, and active in mild alloxan-induced diabetes but inactive in intense alloxan diabetes (nearly all β-cells are destroyed) (Davis & Granner, Citation1996). As these effects require a functional pancreas, it can lower blood sugar levels in non-diabetic and diabetic subjects that are non-insulin dependent (Zhang & Tan, Citation2000). The tolerance test conducted with alloxan-induced diabetic mice indicated that the active principle(s) present in the extracts were more comparable with glibenclamide as metfomin is known to have no hypoglycemic effect in normal mice (Zhang & Tan, Citation2000). While it is tempting to infer a glibenclamide-type of action, the magnitude of effect in the reduction of glucose level at 480 min () was much higher for the extract (37%) compared with glibenclamide (9%) but not as high as insulin (87%). Other probable factors affecting the hypoglycemic and anti-hyperglycemic activity could be more direct insulin-like effects as reported for Momordica charantia. L. (Cucurbitaceae) (Day et al., Citation1990) and Cuminum nigrum. L. (Apiaceae) (Akhtar & Ali, Citation1985), or a β.-receptor antagonist (Kimura et al., Citation1988).
In conclusion, our results imply that extracts of O. chinensis. contain principle(s) that possibly exert multiple actions involving different mechanisms in exerting hypoglycemic and, anti-hyperglycemic effects. This qualifies O. chinensis. to be added to the growing list of potential sources of drugs for the treatment of diabetes. It is important to remember that this plant is used by local people and, therefore, further pharmacological investigations must take this into account. This study also calls attention to the needs of further biochemical investigations of the plant constituents to access the efficacy of the plant in the treatment of diabetes and to determine the underlying mechanism.
Acknowledgments
We thank the North Eastern Council, Ministry of Home Affairs, Government of India, Shillong, for financial assistance in the form of a project (North Eastern Biodiversity Research Cell) and the Department of Biochemistry, North Eastern Hill University, Shillong, India, for providing facilities.
References
- Akhtar MS, Ali MR (1985): Study of hypoglycemic activity of Cuminum nigrum. seeds in normal and alloxan diabetic rabbits. Planta Med 51: 81–85. [CSA]
- Arky RA (1982): Clinical correlates of metabolic derangements of diabetes mellitus. In: Kozak GP, ed., Complications of Diabetes Mellitus. Philadelphia, W.B. Saunders, pp. 16–20.
- Bailey CJ (1992): Biguanides and non-insulin dependent diabetes mellitus. Diabetic Care 15: 755–772. [CSA]
- Bailey CJ, Day C (1989): Traditional plant medicines as treatment for diabetes. Diabetic Care 12: 553–564. [CSA]
- Davis SN, Granner DK (1996): Insulin, oral hypoglycemic agents and the pharmacology of the endocrine pancrease. In: Goodman LS, Gilman AG, eds., The Pharmacological Basis of Therapeutics, 9th ed. New York, McGraw-Hill, pp. 1487–1517.
- Day C, Cartwright T, Provost J, Bailey CJ (1990): Hypoglycemic effect of Momordica charantia. extracts. Planta Med 56: 426–429. [INFOTRIEVE], [CSA]
- Ghosh MN (1984): Toxicity studies. In: Ghosh MN, ed., Fundamentals of Experimental Pharmacology. Calcutta, Scientific Book Agency, pp. 153–158.
- Harborne JB (1998): Phytochemical Methods. 3rd ed. London, Chapman & Hall, pp. 4–7.
- Ivorra MD, Paya M, Villar A (1988): Hypoglycemic and insulin release effects of tormentic acid, a new hypoglycemic natural product. Planta Med 54: 282–286. [INFOTRIEVE], [CSA]
- Jeng-De Su, Osawa T, Kawakishi S, Namiki M (1988): Tannin antioxidants from Osbeckia chinensis.. Phytochemistry 27: 1315–1319. [CSA], [CROSSREF]
- Karam JH (1982): Pancreatic hormones and anti-diabetic drugs. In: Basic and Clinical Pharmacology, 1st ed. California, Lange Medical Publications, pp. 464–466.
- Kayang H, Kharbuli B, Myrboh B, Syiem D (2005): Medicinal plants of meghalaya. In: Bioprospecting & Ethnopharmacology.. Acta Horticulturae 675: 75–80. [CSA]
- Kessler M, Meier W, Storelli C, Semenza G (1975): The biguanide inhibition of D-glucose transport in membrane vesicles from small intestinal brush borders. Biochem Biophys Acta 413: 444–452. [INFOTRIEVE], [CSA]
- Kilberstis P, Roberts L (2001): The puzzle of complex disease. Science 296: 685–689. [CSA], [CROSSREF]
- Kimura Y, Okuda H, Arichi S (1988): Effects of the extracts of Ganoderma lucidum. on blood glucose level in rats. Planta Med 54: 290–294. [INFOTRIEVE], [CSA]
- Marles RJ, Farnsworth NR (1995): Anti-diabetic plants and their active constituents. Phytomedicine 2: 137–189. [CSA]
- O'Moore-Sullivan TM, Prins JB (2002): Thiazolidinediones and type 2 diabetes: New drugs for an old disease. MJA 176: 381–386. [INFOTRIEVE], [CSA]
- Murthy PS (1995): Medicinal plants in diabetes treatment. Indian J Clin Biochem 10: 52–53. [CSA]
- Rao BK, Giri R, Kesavulu MM, Apparao C (2000): Effect of oral administration of bark extracts of Pterocarpus santalinus. L. on blood glucose level in experimental animals. J Ethnopharmacol 74: 69–74. [CSA]
- Shapiro K, Gong WC (2002): Natural products used for diabetes. J Amer Pharm Assoc 42: 217–226. [CSA], [CROSSREF]
- Shirwaikar A, Rajendran K, Kumar DC (2004): Oral antidiabetic activity of Annona squamosa. leaf alcohol extract in NIDDM rats. Pharm Biol 42: 30–35. [CSA]
- Srinivas K, Rao SS, Rao MEB (2000): Investigation on the anti-diabetic activity of Raphanus sativus. Linn. Indian Drugs 37: 445–447. [CSA]
- Steppan CM, Bailey ST, Bhatt S, Brown EJ, Bannerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA (2001): The hormone resistin links obesity to diabetes. Nature 409: 307–312. [INFOTRIEVE], [CSA], [CROSSREF]
- Syiem D, Gareth S, Khup PZ, Khongwir BS, Kharbuli B, Kayang H (2002): Hypoglycemic effect of Potentilla fulgens. L. in normal and alloxan-induced diabetic mice. J Ethnopharmacol 83: 55–56. [INFOTRIEVE], [CSA], [CROSSREF]
- Venkatash S, Reddy GD, Reddy YSR, Sathyavathy D, Reddy MB (2004): Effect of Helicteres isora. root extract on glucose tolerance in glucose-induced hyperglycemic rats. Fitoterapia 75: 364–367. [CSA], [CROSSREF]
- Wilcock C, Bailey CJ (1990): Sites of metformin-stimulated glucose metabolism. Biochem Pharmacol 39: 1831–1834. [INFOTRIEVE], [CSA], [CROSSREF]
- Zeng X, Fang Z, Ma J (1991): Chemical constituents of Osbeckia chinensis. L. Zhongguo Zhong Yao Za Zhi 16:99–101. [INFOTRIEVE], [CSA]
- Zhang FX, Tan BKH (2000): Effects of an ethanolic extract of Gynura procumbens.on serum glucose, cholesterol and triglyceride levels in normal and streptozotocin-induced diabetic rats. Singapore Medical J 41: 1–7. [CSA]