Abstract
The EtOH, CH2Cl2, and petroleum ether extracts from Morinda lucida. Benth. leaves have been shown to exhibit an in vitro. antiplasmodial activity against a chloroquine-sensitive Plasmodium falciparum. strain with IC50 values 5.7 ± 1.3, 5.2 ± 0.8, and 3.9 ± 0.3 µg/mL, respectively. In vivo., at a daily oral dose of 200 mg/kg body weight, they produced at least 62.5%, 67.5%, and 72.2% reduction of parasitemia in mice infected with Plasmodium berghei berghei., respectively. A bioassay-guided fraction of the most active petroleum ether extract resulted in the isolation of two known triterpenic acids as ursolic acid 1 and oleanolic acid 2. In vitro., 1 and 2 exhibited an antiplasmodial activity with IC50 values of 3.1 ± 1.3 and 15.2 ± 3.4 µg/mL, respectively. In vivo., at a daily dose of 200 mg/kg body weight, they produced 97.7% and 37.4% chemosuppression, respectively. However, all tested samples were inactive in vitro. against chloroquine-resistant Plasmodium falciparum. (K1) at the highest tested concentration of 25 µg/mL.
Introduction
Morinda lucida. Benth. (Rubiaceae) is a medicinal plant growing in many African countries. Its leaves, stembark, and rootbark are used in traditional medicine for the treatment of some parasitic diseases such as trypanosomiasis, schistosomiasis, and malaria (including fevers) (Oliver-Bever, 1986; Kambu, 1990; Neuwinger, 2000). Anthraquinones isolated from the root have been reported to exhibit an antifungal (Rath et al., Citation1995) and antiplasmodial (Koumaglo et al., Citation1992) activity. Although some extracts from the leaves, particularly the petroleum ether extract, were previously reported to possess both interesting in vitro. and in vivo. antiplasmodial activity, the active principles of the leaves are still unknown (Obih & Makinde, Citation1985; Awe & Makinde, Citation1998a, b; Tona et al., Citation1999). A 50% methanol extract from the leaves has been found to inhibit Trypanosoma. infections in mice (Asuzu & Chineme, Citation1990), and an ethanol extract possessed antihepatotoxic and purgative effects in mice (Udem et al., Citation1997). The antihepatotoxic activity of the extract would be due to the presence of ursolic acid because this compound isolated from Eucalyptus teretocornis. has been shown to exhibit a dose-dependent hepatoprotective activity in rats (Bunduja Saraswat et al., 1996). Adewinmi and Adesogan (Citation1984, 1986) have tested anthraquinones and an iridoid named oruwacin from the leaves as possible agents in fascioliasis and schistosomiasis control; leaf extracts also possessed antitrypanosomal activity (Asuzu & Chineme, Citation1990).
The current investigation deals with a bioassay-guided isolation of antimalarial active constituents from the petroleum ether extract of M. lucida. leaves.
Materials and Methods
Plant material
The leaves of Morinda lucida. Benth. (Rubiaceae) were collected in Kinshasa, Democratic Republic of Congo, in August 1997. The plant was identified by Mr. M. Nlandu of the Institut Natinial d'Etudes et de Recherches en Agronomie (INERA) of the Uninversity of Kinshasa where a voucher specimen P170897 Nl has been deposited. The plant material was dried at room temperature and reduced to powder.
Extraction and bioassay-guided purificationof the extract E1
Dried powdered leaves (200 g) were macerated and percolated with petroleum ether. The macerate and percolate of each solvent were combined separately and evaporated in vacuo. to give a dried extract denoted as E1 (11.9 g). The EtOH and CH2Cl2 extracts were also obtained in the same manner and denoted as E2 (55.87 g) and E3 (9.66 g) respectively.
An amount of the extract E1 (8 g) was submitted to column chromatography (120 × 3 cm) on silica gel Merck (230–400 mesh) eluted with CH2Cl2/MeOH mixtures of increasing polarity. Several fractions of 20 mL were collected and analyzed by TLC on silica gel Merck (layer thickness 0.25 mm) using n.-hexane-MeOH (9:1) as the mobile phase. The spots were visualized under UV at 254 and 366 nm. After spraying the reagent, the plate was heated at 120°C during 10 min to maximalize colors of spots. Fractions were combined in 15 subfractions according to their chromatographic pattern. Subfractions 1–6 and 10–15 were found to be inactive against chloroquine-sensitive P. falciparum. strain (IC50 > 100 µg/mL). Subfractions 7 and 8 (163 mg) exhibited an antiplasmodial activity with IC50 values less than 5 µg/mL comparable with that of the initial petroleum ether extract, while subfraction 9 showed an antiplasmodial activity with IC50 value of 10 µg/mL. Subfractions 7 and 8 were combined because they contained the same two major components and subfraction 9 was neglected because of its low amount and complex (14 mg). The combined active subfractions were submitted to repetitive preparative TLC on silica gel Merck (layer thickness 0.5 mm) using the same mobile phase as described above resulting in the isolation of two white compounds 1 (35 mg) and 2 (10.3 mg) ().
In vitro. antiplasmodial testing
Throughout the isolation procedure, the antiplasmodial activity was monitored using a chloroquine-sensitive strain of Plasmodium falciparum. from infected human blood as described by Tona et al. (Citation1999). All M. lucida. samples (test concentrations of 1–25 µg/mL) were also tested against a chloroquine-resistant strain of Plasmodium falciparum. (K1). The parasites were continuously cultured according the method described by Trager and Jensen (Citation1976). All experiments were performed in triplicate. Quinine · 2 HCl was used as a reference antiplasmodial compound. The results are expressed as the average IC50 derived from the dose-response curves.
In vivo. antiplasmodial activity
All extracts and isolated compounds from M. lucida. leaves were assessed for their potential in vivo. activity in a 4-day suppressive test against Plasmodium berghei berghei. infections in mice (Tona et al., Citation2001). The ANKA strain of P. berghei berghei. was obtained from the Institute of Tropical Medicine of Antwerp, Belgium. A standard innoculum of 1 × 107 of parasitized erythrocytes from a donor mouse was used to infect each mouse intraperitoneally. The extract and compounds were dissolved in ethanol 10% and then given as a daily oral dose of 200, 400, or 800 mg dry matter/Kg body weight (0.2 mL of solution/mouse). Quinine · 2HCl (10 mg/kg in water) was used as positive control. Thirty mice (25 g body weight) were divided into groups of three (three mice for each dose of extract or compound, and two controls). The test mice were treated daily from day 0 (immediately after infection) to day 3. From day 0 to day 4, a thin smear was made from a tail-blood sample from each mouse and stained with Giemsa so that the level of parasitemia (as the percent of erythrocytes infected) could be evaluated (50% reduction of the number of schizonts with at least three nuclei, counted in 200 erythrocytes). On day 4, the mean level of the parasitemia in each group of mice was determined so that the percentage chemosuppression could be calculated as: [(A.–B.)/A.] × 100, where A. is the mean parasitemia in the negative-control group and B. the parasitemia in the test group (n = 3).
Statistical analysis
The Student's t.-test was used to test the significance of differences between results obtained for different samples and between results for samples and controls. Statistical significance was set at p. value of 0.05.
Results
Two compounds were isolated from the petroleum ether extract and were identified as ursolic acid 1 and oleanolic and 2. All spectral data for these compounds were in good agreement with those previously reported in literature (Kizu et al., 1985; Houghton & Lian 1986; Lin et al., Citation1987). Results from the in vitro. antiplasmodial testing are summarized in . The EtOH, CH2Cl2, and petroleum ether, extracts exhibited an interesting activity with IC50 values ranged from 3 to 6 µg/mL. The activities of the EtOH and CH2Cl2 extracts were similar (p > 0.05) but lower than that of the petroleum extract (p < 0.05). Sufractions F7 and F8 from the column chromatography of the petroleum ether extract exhibited an antiplasmodial activity with IC50 values less than 5 µg/mL, while F9 displayed an IC50 value of 10 µg/mL. Compound 1 exhibited a pronounced antiplasmodial activity with IC50 value of 3.1 ± 1.3 µg/mL. It was more active than 2 (IC50 = 15.2 ± 3.4 µg/mL) against a chloroquine-sensitive P. falciparum. strain (p < 0.001). Unfortunately, the petroleum ether extract compounds 1 and 2 were found to be inactive against chloroquine-resistant P. falciparum. (K1) strain at the highest test concentration of 25 µg/mL.
Table 1. Antimalarial activity of extracts and isolated compounds from Morinda lucida. leaves.
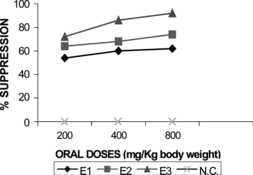
In vivo., at a daily oral dose of 200 mg/kg, the EtOH, CH2Cl2, and the petroleum ether extracts produced 62.5 ± 3.4%, 67.5 ± 2.7%, and 76.2 ± 2.1% of chemosuppression of parasitemia in mice infected with Plasmodium berghei berghei., respectively. Administration of doses of 400 and 800 mg/kg resulted in a dose-dependent effect (). Subfractions F7 and F8 from the column chromatography of the petroleum ether extract produced 85% and 86% reduction of parasitemia in infected mice, respectively. Compound 1 produced even greater reduction in parasitemia of 97.7%. Although 2 only showed 37.5% chemosuppressions, this was still significant compared with the negative control.
Discussion
Some investigations conducted on the petroleum extract from M. lucida. leaves since 1985 have demonstrated that this extract possessed a good in vivo. (Obih et al., Citation1985, Tona et al., Citation1999) as well as an in vitro. antiplasmodial activity (Awe & Makinde, Citation1998a; Tona et al, Citation1999), but no active constituents were reported before the current study. Interestingly, Makinde et al. (Citation1994) showed that the in vivo. antiplasmodial activity of the extract from the plant material collected in Ibadan (Nigeria) showed a seasonal variation depending on the time and the locality its collection. This finding suggested that the large differences in antiplasmodial potency of various samples may have resulted from the reduction in levels of active constituents due to these factors. In the same way, Awe and Makinde (Citation1998b) obtained four fractions from petroleum ether extracts of the leaves collected at different times in Ibabdan. Fractions from the sample collected in March showed a higher in vivo. schizonticidal activity than that collected in June. In addition, de Madureira et al. (2002) have reported the in vitro. antiplasmodial activity of different fractions from the partition of a 70% EtOH extract of the leaves with solvents of different polarities and showed that the petroleum ether fraction possessed weak activity against chloroquine-resistant P. falciparum. (Dd2) with IC50 value of 50 µg/mL and against hepatic development of P. berghei. in Hep G2 liver cells (hepatic schizonticidal activity) with IC50 values ranging from 75 to 170 µg/mL.
In the current investigation, a bioassay-guided fraction of the most active extract (petroleum extract) has resulted in the isolation and identification of two known triterpenoid acids, ursolic acid 1 and oleanolic acid 2. All tested samples were found to be inactive against a chloroquine-resistant P. falciparum. (K1) strain at the highest test concentration of 25 µg/mL. This is qualitatively in agreement with Steele et al. (Citation1999), who have determined the IC50 value of ursolic acid as 36.5 ± 2.2 µg/mL and of oleanolic as 88.8 ± 6.1 µg/mL. Suksamram et al. (2003) reported these compounds to be inactive.
With regard to the in vivo. activity of the petroleum ether extract, our results are qualitatively in good agreement with those previously reported by Obih et al. (Citation1985), or with Awe and Makinde (Citation1998b), who have evaluated the activity of the same extract in rabbits. Results from the current investigation clearly demonstrate a good correlation between the in vitro. and in vivo. antiplasmodial activity of the tested samples. With regard to the in vivo. activity of ursolic acid isolated from Spathodea campanulanta. stembark, our results are qualitatively in good agreement with those reported by Amusan et al. (Citation1996). To our knowledge, this is the first report of the in vivo. antiplasmodial activity of oleanolic acid.
Some triterpenoid acids isolated from Gardenia saxatilis. have been reported to exhibit an interesting antiplasmodial activity against chloroquine-resistant P. falciparum. (K1) strain with IC50 values ranging from 1 to 4 µg/mL (Suksamrarn et al., Citation2003) suggesting that structural differences in this class of compounds play an important role for the manifestation of the activity. Ursolic acid and oleanolic acid isolated from the leaves of Rosmarinus officinalis. have also been reported to exhibit a trypanocidal activity against Trypanosoma cruzi. with different potencies to stop the movement of the parasite (Abe et al., Citation2002), thus, confirming their potential antiparasitic effects. In addition, ursolic and oleanolic acid are reported to possess various interesting pharmacological activities (Liu, Citation1995, 2005). Ursolic acid is reported to be well tolerated in the body. It is nontoxic when fed to guinea-pigs, rats, chickens, and rabbits at levels of 1000 to 5000 mg/kg body weight per day and to humans at a dose of 20 mg kg−1 day−1. At an oral dose of 1 g/kg, oleanolic acid does not cause mortality in rats, and no abnormalities in brain, heart, lung, liver kidney, spleen, or intestine were observed in humans at oral doses of 80 mg/kg (Liu, Citation1995).
Apart from the apolar petroleum ether extract from M. lucida. leaves, polar extracts of the same plant material were also investigated for their potential antimalarial activity in vitro. and/or in vivo. and were reported to be active (Tona et al., Citation1999; de Madureira et al. 2002; Okpekon et al., Citation2004), but no active constituents were isolated. The 80% MeOH extract from the leaves has been reported to exhibit anti-inflammatory, analgesic, and antipyretic activities and may act in a similar manner as the nonsteroidal anti-inflammatory drugs (NSAIDs), which exhibit all these activities (Awe et al., Citation1998). These activities are known to be beneficial for a patient with malaria.
In conclusion, the in vitro. and in vivo. antiplasmodial activity of the petroleum ether extract from M. lucida. leaves can be attributed at least in part to the presence of ursolic acid.
References
- Abe F, Yamauchi T, Nagao T, Kinjo J, Okabe H, Higo H, Akahane H (2002): Ursolic acid as a trypanocidal constituents in Rosemary. Biol Pharm Bull 25: 1485–1487. [INFOTRIEVE], [CSA]
- Adewinmi CO, Adesogan EK (1984): Antraquinones and oruwacin from Morinda. lucida. as possible agents in fascioliasis and schistosomiasis control. Fitoterapia 55: 259–263. [CSA]
- Adewnimi CO, Adesogan EK (1986): Some Nigerian plants used in schistosomiasis control. I. The effect of molluscicides on Molluscan. hearts. Fitoterapia 57: 353–358. [CSA]
- Amusan OOG, Adesogan EK, Makide JM (1996): Antimalarial active principles of Spathodea campanulata. stem bark. Phytother Res 10: 692–693. [CSA], [CROSSREF]
- Asuzu L, Chineme CN (1990): Effects of Morinda lucida. leaf extract on Trypanosoma brucei. infection in mice. J Etnopharmacol 30: 307–313. [CSA], [CROSSREF]
- Awe SO, Makinde JM (1998a): Effect of petroleum ether fraction of Morinda lucida. on Plasmodium berghei berghei. in mice. Pharm Biol 36: 301–304. [CSA]
- Awe SO, Makinde JM (1998b): Evaluation of sensitivity of Plasmodium falciparum. to Morinda lucida. leaf extract using rabbit in vitro. microtest technique. Indian J Pharmacol 30: 51–53. [CSA]
- Awe SO, Olajide OA, Adeboye JO, Makinde JM (1998): Some pharmacological studies on Morinda lucida.. Indian J Pharmacol 30: 38–42. [CSA]
- Bunduja Saraswat PKS, Vissen R, Dayal R, Agrawal DP, Patnak GK (1996): Protective action of ursolic acid against chemical induced hepato-toxicity in rats. Indian J Pharmacol 28: 232–239. [CSA]
- de Madureira MC, Martins AP, Gomes M, Paiva J, da Cunha AP, do Rosário V (2002): Antimalarial activity of medicinal plants used in tradtional medicine in S. Tomé and Principe Islands. J Ethnopharmacol 81: 23–29. [CSA], [CROSSREF]
- Houghton PJ, Lian LM (1986): Triterpenoids from Desfontaina spinosa.. Phytochemistry 25: 1939–1944. [CSA], [CROSSREF]
- Kambu K (1990): Eléments de Phytothérapie Comparés. Plantes Médicinales Africaines. Kinshasa, CRP, p. 59–61.
- Kizu H, Kitayama S, Nakatani F, Tomimori T, Namba T (1985): Studies on Nepalese crude drugs. III. On the saponins of Hedera nepalensis. K. Koch. Chem Pham Bull 33: 3324–3329, [CSA]
- Koumaglo K, Gbeassor M, Nkabou CL, de Souza CC, Wenee W (1992): Effects of three compounds extracted from Morinda lucida. on Plasmodium falciparum.. Planta Med 58: 533–534. [INFOTRIEVE], [CSA]
- Lin CN, Chung MI, Gan KH, Chang JR (1987): Xanthones from Formasan Gentianaceous plants. Phytochemistry 26: 2381–2384. [CSA], [CROSSREF]
- Liu J (1995): Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol 49: 57–68. [INFOTRIEVE], [CSA], [CROSSREF]
- Liu J (2005): Oleanolic acid and ursolic acid research perspectives. J Ethnopharmacol 100: 92–94. [INFOTRIEVE], [CSA], [CROSSREF]
- Makinde JM, Awe SO, Salako LA (1994): Seasonal variation in the antimalarial activity of Morinda lucida. on Plasmodium berghei berghei. in mice. Fitoterapia 61: 125–130. [CSA]
- Neuwinger HD (2000): African Traditional Medicine. A Dictionary of Plant Use and Applications. Stuttgart, Medpharm Scientific, p.342–343. [CSA]
- Obih PO, Makinde JM, Laoye J (1985): Investigation of various extracts of Morinda. lucida. for antimalarial actions on Plasmodium berghei berghei.. J Med Med Sci 144: 45–49. [CSA]
- Oliver-Bever B (1986): Medicinal Plants in Tropical West Africa, 1st ed. Cambridge, Cambridge University Press, p. 44.
- Okpekon T, Youlu S, Gleye C, Roblot, F, Loiseau P, Bories C, Grellier P, Frappier F, Laurens A, Hocquemiller R (2004): Antiparasitic activities of medicinal plants used in Ivory Coast. J Ethnopharmacol 90: 91–97. [INFOTRIEVE], [CSA], [CROSSREF]
- Rath G, Ndozao M, Hostettman K (1995): Antifungal antraquinones from Morinda lucida.. Int J Pharmacog 73: 107–114. [CSA]
- Steele JC, Warhurst DC, Kirby GC, Simmonds MSJ (1999): In vitro. and in vivo. evaluation of betulinic acid as antimalarial. Phytother Res 13: 113–119. [CSA], [CROSSREF]
- Suksamrarn A, Tanachatchairatana T, Kanokmedhakul T (2003): Antiplasmodial triterpenes from twigs of Gardenia saxatilis.. J Ethnopharmacol 88: 275–277. [INFOTRIEVE], [CSA], [CROSSREF]
- Tona L, Ngimbi, NP, Tsakala M, Mesia K, Cimanga K, Apers S, De Bruyne T, Pieters L, Totté J, Vlietinck AJ (1999): Antimalarial activity of 20 crude extracts from nine African medicinal plants used in Kinshasa, Congo. J Ethnopharmacol 66: 193–203. [CSA], [CROSSREF]
- Tona L, Mesia K, Ngimbi, NP, Chirimwami B, Okond'ahoka A, Cimanga K, De Bruyne T, Apers S, Hermans N, Totté J, Pieters L, Vlietinck AJ (2001): In vivo atimalarial activity of Cassia occidentalis., Morinda morindoides. and Phyllanthus niruri.. Ann Trop Med Parasitol 95: 47–57. [INFOTRIEVE], [CSA], [CROSSREF]
- Trager W, Jensen JB (1976): Human malaria parasites in continuous culture. Science 193: 673–675. [INFOTRIEVE], [CSA], [CROSSREF]
- Udem SU, Madubunyi DL, Okoye JOA, Anra SM (1997): Anti-hepatotoxic effects of the ethanolic extracts of Combetum dolichopetalum. root bark and Morinda. lucida. leaf. Fitoterapia 58: 21–25. [CSA]