Abstract
This study was undertaken to evaluate the effect of topical administration of Rubus suavissimus. S. Lee (Rosaceae) (RUS), an extract of Chinese sweet leaf tea, in the prevention of experimentally induced corneal neovascularization. Corneal neovascularization was induced in 40 rat eyes (1 per animal) by a silver nitrate cauterization technique. Three concentrations (0.1%, 1%, and 10% w/v) of RUS in saline were administered topically twice a day for 7 days. The coverage of the corneal surface by neovascular vessels was measured, using photographs, as a percentage of total cornea area. A histopathologic score was assigned to each cornea. The difference in mean burn stimulus scores of treatment and placebo groups was not statistically significant (p = 0.714). The 10% RUS solution caused a significant decrease in the percentage of neovascularization in response to cauterization (p < 0.01); the 0.1% and 1% concentrations were not significantly different from controls (p = 0.173). The 10% concentration group had significantly less neovascularization than the control group (p < 0.01). It was found that topical application of 10% RUS solution caused a significant decrease in neovascular response to silver nitrate cauterization compared with control eyes.
Introduction
Corneal neovascularization is a severely disabling condition resulting in loss of the immunologic privilege of the cornea and causing visual impairment, depending on the extent of the vascular invasion. In addition, penetrating keratoplasty carries an increased risk of failure in cases with corneal neovascularization (Epstein et al., Citation1987).
Conventional therapy for corneal neovascularization relies on the use of topical corticosteroids. However, the use of steroids may contribute to cataract formation, microbial keratitis, and glaucoma (Suzuki et al., 2002). Recognition of the potential benefits of controlling angiogenesis has led to a search for natural and/or synthetic angiogenesis inhibitors (Crum et al., Citation1985; Folkman et al., Citation1988; Fotsis et al., Citation1995; Woltering et al., Citation1997; Benelli et al., Citation1998; Bocci et al., Citation1999; Joussen et al., Citation1999Citation2001). Among the natural inhibitors of angiogenesis was the Chinese sweet leaf tea (Rubus suavissimus. S. Lee [RUS]) determined in a human tissue-based angiogenesis assay (Liu et al., Citation2006). RUS has shown folk medicinal values in maintaining healthy kidneys, blood pressure, and blood sugar metabolism (Huang & Jiang, Citation2002). Scientific investigations found RUS to be antiinflammatory and anti-allergic (Nakahara et al., Citation1996; Kotaro, Citation1997; Nakahara, Citation1998; Ono, Citation2002) as well as inhibitory to NF-κB (Liu et al., Citation2005).
The main objective of this study was evaluate of the effect of topical administration of a RUS extract in the prevention of experimentally induced corneal neovascularization.
Materials and Methods
Experimental animals
Male Long-Evans pigmented rats weighing approximately 200 g were used in this study. All animals were housed in individual cages and maintained under standard conditions. The experimental protocol was approved by the Institutional Animal Care and Use Committee of Tulane University Health Sciences Center. Forty eyes of 40 rats were used in a study of the topical administration of a RUS extract or placebo. One eye of each animal served as a treated eye and the other served as a nontreated control eye.
Preparation of RUS extract
The leaves of R. suavissimus. S. Lee (Rosaceae) were collected from a farm in Guangxi, People's Republic of China, and authenticated by Jian Zhang of Guangxi Normal University. The leaves were air-dried, and stored at room temperature before extraction. Dried leaves (100 g) were soaked in 2000 mL water for 1 h, brought to a boil for 30 min, cooled to room temperature, and reboiled for 30 additional min. The water extract was subsequently filtered with cheesecloth and spray-dried to obtain 31 g of a crude extract powder. The powder was diluted with saline and sterile filtered before use.
Induction of corneal neovascularization
To induce corneal neovascularization in rats, a silver nitrate cauterization technique described by Mahoney and Waterbury (Citation1985) was used. All procedures were performed under general anesthesia induced by intraperitoneally administered ketamine hydrochloride and xylazine combination (94.7 mg/kg body weight). One drop of 0.5% topical proparacaine was applied to each cornea before the procedure. All corneas were cauterized by pressing the applicator stick (diameter 1.8 mm) coated with 75% silver nitrate and 25% potassium nitrate to the central cornea for 8 s under the operating microscope. Excess silver nitrate was removed by rinsing the eyes with 10 mL of a balanced salt solution and then gently blotting them with tissue paper. To increase the reproducibility of the injuries, one investigator cauterized all animals. After cauterization, the rats were randomized to eliminate any potential bias in the degree of injury within the different groups.
The rats were divided into four groups. Group 1 (n = 10) received 0.1% topical RUS, group 2 (n = 10) 1% topical RUS, group 3 (n = 10) 10% topical RUS, and group 4 (n = 10) saline; all doses were topically administered two-times a day for 7 days. Treatment started immediately after cauterization in all groups.
All animals were anesthetized as described above and their corneas evaluated by slit-lamp microscopy. Corneal photographs were taken with × 25 magnification using a digital camera attached to the slit-lamp microscope (Topcon SL-7E, Japan). Neovascularization in each cornea was evaluated using the technique described by Mahoney and Waterbury (Citation1985) by an examiner who was blinded to the treatment groups to minimize the observer bias. For each eye, the extent of burn stimulus response was scored as: 0 (no blister, not raised above corneal surface), +1 (small blister, raised slightly above the surface), +2 (medium blister, raised moderately above the surface), +3 (large blister). The corneal surface covered with neovascular vessels was measured on the photographs as the percentage of the total area of the cornea. Image analysis was performed semiautomatically on each cornea using an image processing and analysis software program (BS200D-Image Analysis Software BAB Bilgisayar ve Ticaret Ltd., Ankara, Turkey). The area of neovascularization was measured in terms of pixels, and its ratio to the entire corneal area was determined as the percentage of corneal neovascularization.
Only the corneas with a burn stimulus score of +1 or higher were included for the calculation of the mean burn stimulus and neovascularization scores in each group. Percent inhibition was calculated by comparing the mean percentage of neovascularization in each drug-treated group to that in the control group. After scoring the burn stimulus and the percentage of neovascularization for all groups, the animals were sacrificed on the seventh day.
Tissue preparation/histopathology
After sedation using the intraperitoneally administered ketamine hydrochloride and xylazine combination (94.7 mg/kg body weight), enucleation was performed before the animals were euthanized. Immediately after enucleation, the globes were penetrated with a 27-gauge needle, 1.0 mm from the limbus at the 3 and 9 o'clock meridians to allow the fixative to fill the eyes rapidly. The eyes were prepared for histologic examination using 2% paraformaldehyde, 3% glutaraldehyde fixative. After fixation for 24 h, the eyes were removed from the fixative and corneas were dehydrated and sectioned. For preinfiltration, ethanol and Technovit 7100 were used, and the eyes were infiltrated overnight using Technovit 7100. The tissues were embedded in methacrylate overnight and cut at 3-µm intervals, then stained with 1% Toluidine blue for light microscopy.
Light microscopic examination was performed on every microscopic section. Sections were examined by dividing the corneas into two halves through the center of the lesion and were evaluated with regard to the intensity of new vessels, polymorphonucleated (PMN) leukocytes, edema, and fibroblastic activity and were scored as 0 (no change), +1 (mild), +2 (moderate), +3 (severe activity). An average histopathologic score for each cornea was calculated.
Statistical analysis
Statistical analyses were performed using each rat as an experimental unit with Statistical Analysis System (SAS, SAS version 8.02, Cary, NC, USA) software. One-way analysis of variance (ANOVA) was conducted, and treatment means were separated at p ≤ 0.05 with the least significant difference (LSD) test. A regression analysis was performed to determine the rate of change in corneal neovascularization after topical application of RUS extract. A p value of ≤ 0.05 was considered significant.
Results
The burn stimulus, percentage of neovascularization, and histopathologic scores of each cornea in the treatment and placebo groups are shown in . The burn stimulus score was +1 or higher in all eyes. The mean burn stimulus score did not show a statistically significant difference between the treatment and the placebo groups (p = 0.714). By gross examination, all eyes treated with RUS showed less inflammation during the treatment period with less eyelid edema and less ciliary injection. shows the average burn stimulus, percentage of neovascularization, histopathologic scores, and inhibition percentage of test drugs on the neovascularization response in comparison with the controls for each group. Topical application of 10% RUS solution caused a significant decrease in the percentage of neovascularization in response to silver nitrate cauterization (p < 0.01), whereas topical administration of the 0.1% and 1% concentrations of RUS extract showed no significant difference compared with control eyes (p = 0.173) (). In drug-treated and placebo eyes, the severity of burn stimulus response was positively correlated with the extent of neovascular growth. Histopathologic evaluation of group 3 (10% concentration) showed significantly less neovascularization compared with the control group (p < 0.01) ().
Table 1.. Inhibition of neovascularization by the topical application of Rubus suavissimus. (RUS) extract.
Figure 1 Photographs of the rat corneas 7 days after silver nitrate cauterization showing new vessels invading the cornea (magnification, × 16). Note the degree of neovascularization, after topical isotonic saline (control) (A), after topical treatment with 0.1% RUS (B), after topical treatment with 1% RUS (C), and after topical treatment with 10% RUS (D).
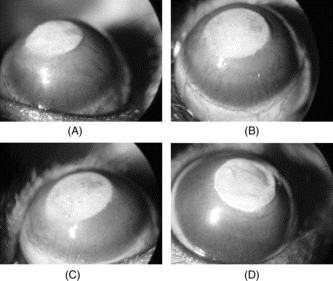
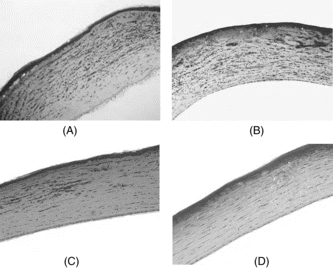
Figure 2 Histologic observations depicting the effects of topical RUS on corneal inflammation of rat corneas 7 days after silver nitrate cauterization. Note the intensity of new vessels, polymorphonucleated (PMN) leukocytes, edema, and fibroblastic activity after topical isotonic saline (control) (A), after topical 0.1% RUS (B), after topical 1% RUS (C), and after topical 10% RUS (D). Notice the lesser amount of fibroblastic activity with decreased number of PMN cells and new vessels in the 10% RUS group.
Discussion
In this study, our results with the highest concentration (10%) of topical RUS in the prevention of corneal neovascularization were encouraging. Topical application of the 10% RUS solution caused a significant decrease in neovascular response to silver nitrate cauterization and also showed a significantly lower intensity of neovascularization compared with control eyes. Isolation of an active ingredient or fraction from the crude RUS extract may significantly enhance the efficacy of this therapy.
Acknowledgment
Proprietary interest: Louisiana State University has filed a patent on this extract and its active ingredients as inhibitors of angiogenesis.
References
- Benelli U, Bocci G, Danesi R (1998): The heparan sulfate suleparoide inhibits rat corneal angiogenesis and in vitro. neovascularization. Exp Eye Res 67: 133–142.
- Bocci G, Danesi R, Benelli U (1999): Inhibitory effect of suramin in rat models of angiogenesis in vitro. and in vivo.. Canxer Chemother Pharmacol 43: 205–212.
- Crum R, Szabo S, Folkman J (1985): A new class of steroids inhibits angiogenesis in the presence of heparin or a heparin fragment. Science 230: 1375–1388.
- Epstein RJ, Stulting RD, Hendricks RL, Harris DM (1987): Corneal neovascularization. Pathogenesis and inhibition. Cornea 6: 250–257.
- Folkman J, Klagsbrun M, Sasse J, Wadzinski M, Ingber D, Vlodavsky I (1988): A heparin-binding angiogenic protein–basic fibroblast growth factor–is stored within basement membrane. Am J Pathol 130: 393–400.
- Fotsis T, Pepper M, Adlercreutz H, Hase T, Montesano R, Schweigerer L (1995): Genistein, a dietary ingested isoflavonoid, inhibits cell proliferation and in vitro. angiogenesis. J Nutr 125 (Suppl 3): 790S–797S.
- Huang P-F, Jiang S-Q (2002): Comprehensive utilization of Rubus suavissimus. S. Lee. Guangxi Huagong 31: 24–25.
- Joussen AM, Kruse FE, Volcker HE, Kirchhof B (1999): Topical application of methotrexate for inhibition of corneal angiogenesis. Graefes Arch Clin Exp Ophthalmol 237: 920–927.
- Joussen AM, Beecken WD, Moromizato Y, Schwartz A, Kirchhof B, Poulaki V (2001): Inhibition of inflammatory corneal angiogenesis by TNP-470. Invest Ophthalmol Vis Sci 42: 2510–2516.
- Kotaro U (1997): Antiallergy action of Rubus suavissimus.. Shokuhin Kogyo 40: 52–59.
- Liu D, Gao Z, Zhang J, Ye J, Liu Z (2005): Bioassay-guided fractionation of the Rubus suavissimus. leaf extracts possessing NF-κB inhibitory activities and a separable cytotoxicity. Pharm Biol 43: 713–717.
- Liu Z, Schwimer J, Liu D, Lewis J, Greenway FL, York DA, Woltering EA (2006). Gallic acid is partially responsible for the anti-angiogenic activities of Rubus suavissimus. leaf extract. Phytother Res 20: 806–813.
- Mahoney JM, Waterbury LD (1985): Drug effects on the neovascularization response to silver nitrate cauterization of the rat cornea. Curr Eye Res 4: 531–535.
- Nakahara K (1998): Anti-allergic activity of Tiencha and oolong tea polyphenols. Food Style 21 (2): 45–49.
- Nakahara K, Miyagawa K, Kodama T, Fujii W (1996): Anti-allergic composition containing GOD-type ellagitannin as active ingredient. Europe Patent No. 727218.
- Ono Y (2002): The health beneficial effects of Tien-cha (Rubus suavissimus. tea) and its applications. Food Style 21 (6): 77–80.
- Suzuki T, Sano Y, Kinoshita S (2000): Effects of 1α,25-dihydroxyvitamin D3 on Langerhans cell migration and corneal neovascularization in mice. Invest Ophthalmol Vis Sci 41: 154–158.
- Woltering EA, Watson JC, Alperin-Lea RC (1997): Somatostatin analogs: angiogenesis inhibitors with novel mechanisms of action. Invest New Drugs 15: 77–86.