Abstract
3,4-Dihydroxy L-phenylalanine (L-DOPA) is considered a potent drug for the treatment of Parkinson disease, a neurologic disorder. Enantiomerically pure L-DOPA is produced from L-tyrosine in a single-step biotransformation process using callus cultures of the plant Portulaca grandiflora. Hook (Portulacaceae). Callus cultures were induced in MS medium provided with growth regulators such as benzylaminopurine (BA; 1.5 mg L−1) and 2,4-dichlorophenoxyacetic acid (2,4-D; 0.1 mg L−1) and were found to be an excellent source of tyrosinase, which in turn was used for the biotransformation of L-tyrosine into L-DOPA. A culture time of 20–25 days was found to be optimum for biomass production, and the tyrosinase activity in the medium was found to be 2.19 U/mL. Optimization of L-DOPA production was carried out by varying the concentration of BA and 2,4-D. In view of the fact that the enzyme tyrosinase has a dicopper catalytic site, the concentration of Cu2+ was manipulated in the media to study its impact on biotransformation rate. The L-DOPA was purified by column chromatography and the analysis was done by TLC, HPLC, FT IR, and MS. The optimized production of L-DOPA, 48.8 mg L−1 h−1, is one of the highest values recorded in the literature.
Introduction
Tyrosinase (EC 1.14.18.1) is an oxygen oxidoreductase, a deficiency of which causes oculocutaneus albinism in humans. This enzyme catalyzes the o.-hydroxylation of L-tyrosine to yield 3,4-dihydroxy L-phenylalanine (L-DOPA), which is then converted to dopaquinone during its biosynthesis to melanin. Tyrosinase catalyzes the first two of these reactions, namely, the conversion of L-tyrosine into L-DOPA and the conversion of L-DOPA into dopaquinone. In the United States alone, over a million victims of Parkinson disease are reported, and it is reported to cost the nation about $5.6 billion annually. Though different types of treatments are employed for Parkinson disease, a degenerative brain disorder that leads to dementia, L-DOPA is considered the most potent drug available in the market. Even though the chemical synthesis of DOPA is rapid, the resultant racemic DL-mixture is inactive, and further separation of enantiomerically pure L-DOPA from this mixture is very difficult. D-DOPA interferes with the activity of DOPA decarboxylase, the enzyme involved in the production of dopamine in the brain. Hence, a one-step economical bioconversion of L-tyrosine into L-DOPA is highly important.
As the demand for L-DOPA is high, its production by cell cultures is highly relevant (Huang et al., Citation1995). The high cost of the drug can be reduced by either reducing the cost of recovery and purification or by using cheap and easily available sources such as plants for the biotransformation. Previous studies on L-DOPA production mainly concentrated on plants such as Mucuna pruriens. L., M. deeringiana. L. (Fabaceae), banana (Huizing et al., Citation1985; Bapat et al., Citation2000), and so forth. L-Tyrosine is mostly used as a precursor for L-DOPA production because it is inexpensive (Pras et al., Citation1989). Biotransformation has also been done using the purified tyrosinase immobilized in calcium alginate (Wichers et al., Citation1985).
Tyrosinase present in microbes, mainly fungi such as Aspergillus. spp. (belonging to the class Deuteromycetes) is also used for the biotransformation (Ali et al., Citation2002; Khan et al., Citation2004). However, the activity is generally very weak and the L-tyrosine and L-DOPA are rapidly decomposed to other metabolites, thus making the stoichiometric formation of L-DOPA difficult (James & Fling, Citation2001). We have screened a large number of plants and selected the plant Portulaca grandiflora. (Portulacaceae), common name “purselane,” as a potent source of tyrosinase. We were able to successfully induce the callus from the plant and then use suspension cultures as a very cost-effective alternative for the efficient biotransformation of L-tyrosine into L-DOPA. We also studied the impact of growth regulators benzylaminopurine (BA), 2,4-dichlorophenoxyacetic acid (2,4-D), and also Cu2+ concentration (as CuSO4·5H2O) on L-DOPA production.
Materials and Methods
Tissue culture and callus initiation
Leaf and stem explants were washed well in 10% labolene, a detergent, for 10 min before exposing to 0.1% mercuric chloride for 5 min and were inoculated onto media containing different concentrations of hormones on full-strength MS medium (Murashige & Scoog, Citation1962). Sucrose (3.0%) was used as the carbon source and 0.8% (w/v) agar as solidifying agent and incubated at 25°C under a photo period of 12/12 h. BA (1.5 mg L−1) and 2,4-D (0.1 mg L−1) were used for callus initiation and proliferation. Optimum period for biomass production was determined. Subculturing was done after every 20–25 days, and after the third passage, the medium composition was altered to exclude vitamins, amino acids, and micronutrients in order to habituate the callus in biotransformation medium.
Medium for biotransformation
Liquid MS medium lacking vitamins, amino acids, and micronutrients was prepared in order to remove the interference on biotransformation, for the ease of separation and also for reducing the cost. The medium was provided with BA (1.5 mg L−1) and 2,4-D (0.1 mg L−1). Callus (5 g) was suspended in 100 mL medium provided with 5 mM each of L-tyrosine and L-ascorbate dissolved in 0.1 M phosphate buffer (pH 5.8). Cell suspensions thus prepared were kept under constant shaking (140 rpm).
Estimation of L-DOPA formed and L-tyrosine used
Estimation of L-DOPA as well as tyrosine was done by the modification of the procedure reported by Arnow (Citation1937) and Jimenez-Hamman & Saville (Citation1996), and the rate of conversion of L-tyrosine to L-DOPA was monitored spectrophotometrically after every 2 h of incubation. To estimate L-DOPA, 1 mL each of the following reagents was added to 1 mL of culture supernatant: 2 M HCl, 15% (w/v) sodium molybdate, 15% (w/v) sodium nitrite, and 2 M sodium hydroxide. The acid is added to inactivate the residual free enzyme, stopping further biotransformation (Pialis & Saville, Citation1998). Addition of sodium hydroxide turns the yellow color of the reaction mixture to an orange-red color due to diazotization of the amino group of DOPA, and the concentration of DOPA formed was detected at 460 nm in a spectrophotometer (Shimadzu UV 2100, Tokyo, Japan) after 30 min from the standard curve of L-DOPA.
To estimate the amount of L-tyrosine used: To 1 mL of the supernatant from the reaction mixture, 1 mL of 2.5 mM mercuric sulfate reagent was added, placed in a boiling water bath for 10 min, and was then cooled. Sodium nitrite (1 mL) and sodium molybdate (1 mL) were added to develop the color, which was read at 450 nm, and the concentration was obtained from the standard curve (Ali et al., Citation2002).
Extraction and detection of the transformed product
A sample of the medium (100 mL) was lyophilized giving a residue (25.6 mg) that was chromatographed on silica gel (100–120 mesh size in the ratio 1:30) using CHCl3-BuOH-H2O (3:2:1) (v/v) as solvent. Elution of L-DOPA was monitored by TLC in phenol-water system (75:25) (w/v). Spray reagent used 3% ninhydrin in n.-butanol. The fractions with identical spots (showing only L-DOPA as per TLC) were pooled and concentrated under reduced pressure in a rotatory evaporator to get a pure product of melting point 275–278°C.
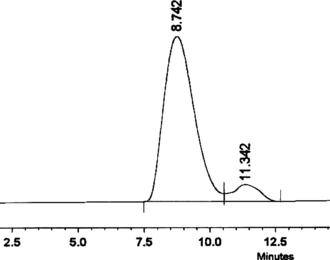
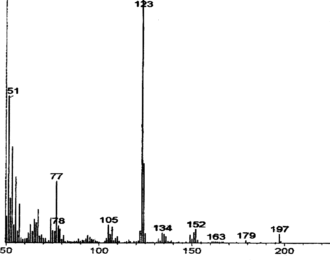
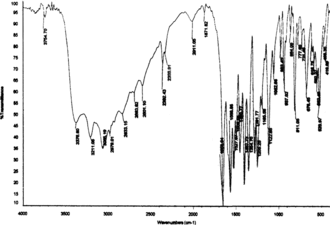
The samples were analyzed by HPLC (Shimadzu LC-8A) using a reverse-phase C18 column (250 × 4.6 mm, particle size 5 µ). Isocratic elution (1 mL/min) was done with the solvent system (1.5% aqueous H3PO4:80% aqueous acetonitrile, 98:2, v/v) at 30±2°C. Five-microliter aliquots of the samples were loaded onto the column, and the separated components were detected at 280 nm using a UV-Vis detector. The product was confirmed by FTIR (BOMEM MB Series, Quebec, Canada) and MS (Hewlett Packard MS no. HP 5995, Loveland, CO, USA) run at 70 eV, by the DIP (direct injection port) method, and the levorotatary nature was identified (JASCO Digital model DIP-370 polarimeter, Tokyo, Japan).
Impact of the plant growth regulators BA and 2,4-D on L-DOPA formation
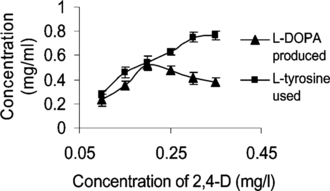
In order to find out the influence of BA on biotransformation, the concentration was altered from 0.5 to 5.0 mg L−1, keeping 2,4-D a constant (0.1 mg L−1). The effect of 2,4-D concentrations (0.05–0.35 mg L−1) was also studied keeping the concentration of BA at optimum (4.5 mg L−1).
Effect of Cu2+ concentration on biotransformation
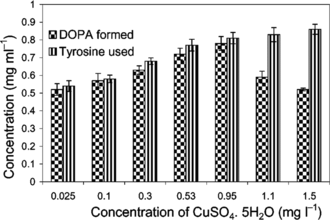
In order to study the effect of different concentrations of Cu2+, 0.025 to 1.5 mg L−1 of CuSO4·5H2O were provided in the medium, keeping BA (1.5 mg L−1) and 2,4-D (0.1 mg L−1) constant.
Results and Discussion
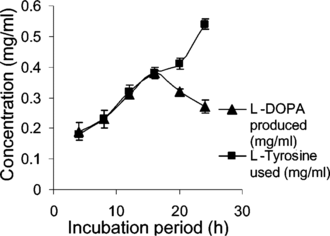
In the current study, maximum biomass was produced on day 25 after inoculation with a hormone combination of BA (1.5 mg L−1) and 2,4-D (0.1 mg L−1). Tyrosinase activity was found to be 2.19 U/mL of the medium (at day 25) and 16 h was found to be optimum for L-DOPA production () under the specified cultural conditions. The pH was maintained at 5.8 as it was found favorable for both the plant cells and L-DOPA, of which the latter is readily oxidized at alkaline pH and at high temperature (Siddhuraju & Becker, Citation2001). Biosynthesis of L-DOPA is much better in terms of production rate as well as time duration compared with most other reports (). In addition to the role as a precursor of L-DOPA, L-tyrosine is reported to function as an inducer for tyrosinase activity and also for DOPA production (Musso et al., Citation1997; Katayama et al., Citation2000). Biotransformations in other systems have reported the conversion of L-DOPA to other metabolites along with changes in the metabolic pathway of the precursor tyrosine for protein synthesis (Obata-Sasamoto et al., Citation1981). Unwanted oxidation products of L-DOPA were lacking in the reaction mixtures, as P. grandiflora. shows very good tyrosine hydroxylase activity (Steiner et al., Citation1996) and the concentration of L-ascorbic acid (2.5 mM) supplied in the medium was enough to prevent further oxidation of DOPA. However, after a period of 16 h, DOPA was found to be converted to other metabolites, and the concentration of L-ascorbic acid was fixed at 2.5 mM as higher concentrations inhibited tyrosinase activity (results not shown). The transformation medium was devoid of a large number of nutrients, but it did not affect either growth or the biotransformation rate, because the suspension culture was acclimatized previously. L-DOPA (Rf. 0.39) and L-tyrosine (Rf. 0.53) were completely separated by silica gel TLC and located with ninhydrin reagent. Ascorbic acid, used to prevent further oxidation of L-DOPA, did not give interfering spots. HPLC analysis showed differential rates of biotransformation depending on the reaction time. The retention time for L-DOPA was 8.5 to 9.5 min and for L-tyrosine it was 11 to 13 min. shows sample showing more than 90% conversion after a period of 14 h. MS () and IR () data were in concordance with the authentic samples. The molecular ion at 197 and its daughter fragments clearly indicated that the product formed by the bioconversion is DOPA. The MS data were 197 (M+), 179 (M+-18) (4.12), 152 (6.2), 134 (4.1), 105 (8.3), 77 (25.8), 51 (60.8), 123 (100%). IR (KBr) Vmax-3754, 3377, 3211, 3065, 2833, 1653, 1567, 1527, 1408, 1355, 1255, 1123, 937, 811, 678, 552, 526, 466 cm−1. Specific rotation of the L-DOPA obtained was [α]D25 = − 11.7°, confirming the levorotatory nature of the product (L-DOPA).
Table 1.. Comparison of L-DOPA production by biological methods.
Effect of the growth regulators BA and 2,4-D on L-DOPA production
Though BA showed very little influence on the rate of conversion, an optimum of 24.4 mg L−1 h−1 L-DOPA was produced when the concentration of BA supplied was increased to 4.5 mg L−1 (at constant 2,4-D 0.1 mg L−1). But 2,4-D was found to favor L-DOPA production up to a concentration of 0.25 mg L−1 (). An optimum rate of 32.5 mg L−1 h−1 was obtained when the concentration of 2,4-D was 0.2 mg L−1. Further increase in the hormone led to a decrease in the production, which may be due to the inhibitory effect of the hormone on callus growth. Reduction in biotransformation rate at high 2,4-D concentrations is also reported by Wichers et al. (Citation1993). Higher concentrations of cytokinin and suboptimal conditions of 2,4-D are reported to be good for the production of L-DOPA (Obata-Sasamoto et al., Citation1981).
Effect of Cu2+ on biotransformation
The biotransformation rate was doubled (48.8 mg L−1 h−1) when the concentration of CuSO4·5H2O was increased to 0.95 mg L−1, whereas the basal MS medium is provided with only 0.025 mg L−1 of CuSO4·5H2O (). The increase in biotransformation rate in the presence of higher Cu2+ can be attributed to the enhanced production and activity of the tyrosinase that has a dicopper catalytic site. However, further increase in Cu2+ concentration (above 1.0 mg L−1) resulted in reduced biomass growth.
Conclusions
Biotransformation of L-tyrosine into L-DOPA using Portulaca grandiflora. suspension culture was found to be one of the best and most cost-effective methods for efficient and rapid production of L-DOPA (optimum rate 48.8 mg L−1 h−1). The biotransformation medium was devoid of vitamins, amino acids, and micronutrients. Concentrations of 2,4-D up to 0.25 mg L−1 favored L-DOPA formation. When CuSO4·5H2O concentration was increased to 0.95 mg L−1, the biotransformation rate was doubled. Recovery of the transformed product was easier than that of the microbial systems. The possibility of continuous production of L-DOPA is very envisaged.
Acknowledgment
The first author acknowledges CSIR, India, for the award of Senior Research Fellowship.
References
- Ali S, Haq I, Qadeer MA (2002): Novel technique for microbial production of 3,4-dihydroxy phenyl L-alanine by a mutant strain of Aspergillus oryzae.. Electronic J Biotech 5: 118–123.
- Arnow LE (1937): Colorimetric determination of the components of 3,4-dihydroxy phenyl alanine–tyrosine mixtures. J Biol Chem 118: 531–537.
- Bapat VA, Suprasanna P, Ganapathi TR, Rao PS (2000): In vitro. production of L-DOPA in tissue cultures of banana. Pharm Biol 38: 271–273.
- Chattopadhyay S, Datta SK, Mahato SB (1994): Production of L-DOPA from cell suspension culture of Mucuna pruriens f. pruriens.. Plant Cell Rep 13: 519–522.
- Foor F, Morin N, Bostian KA (1993): Production of L-dihydroxyphenylalanine in Escherichia coli. with the tyrosine phenol-lyase gene cloned from Erwinia herbicola.. Appl Environ Microbiol 59: 3070–3075.
- Huang S, Chen S, Wu K, Taung W (1995): Strategy for introducing pertinent cell line and optimization of the medium for Stizolobium hassjoo. producing L-DOPA. J Fermentation Bioeng 79: 342–347.
- Huizing HJ, Wijnsma R, Batterman S, Malingre Th M, Wichers HJ (1985): Production of L-DOPA by cell suspension cultures of Mucuna pruriens.. I. Initiation and maintenance of cell suspension cultures of Mucuna pruriens. and identification of L-DOPA. Plant Cell Tissue Organ Cult 4: 61–73.
- James P, Fling G (2001): Tyrosinase activity of different fungal strains. Biochem Biophys 112: 98–103.
- Jimenez-Hamman MC, Saville BA (1996): Enhancement of tyrosinase stability by immobilization on Nylon 6, 6. Trans Inst Chem Eng 74: 47–52.
- Katayama T, Suzuki H, Koyanagi T, Kumagai H (2000): Cloning and random mutagenesis of the Erwinia herbicola tyrR. gene for high-level expression of tyrosine phenol-lyase. Appl Environ Microb 66: 4764–4771.
- Khan A, Ali S, Haq I (2004): Study of cultural conditions for the conversion of L-tyrosine to L-DOPA by a strain of Aspergillus oryzae. Isb-9. J Food Tech 2 (1): 9–18.
- Murashige T, Skoog F (1962): A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497.
- Musso NR, Brenci S, Indiveri F, Lotti G (1997): L-Tyrosine and nicotine induce synthesis of L-DOPA and norepinephrine in human lymphocytes. J Neuroimmunol 74: 117–120.
- Obata-Sasamoto H, Nishi N, Komamine A (1981): Mechanism of suppression of DOPA accumulation in a callus culture of Stizolobium hassjoo.. Plant Cell Physiol 22: 827–835.
- Pialis P, Jimenez-Hamman MC, Saville BA (1996): L-DOPA production from tyrosinase immobilized on Nylon 6,6. Biotech Bioeng 51: 141–147.
- Pialis P, Saville BA (1998): Production of L-DOPA from tyrosine immobilized on nylon 6,6: enzyme stability and scale up. Enzyme Microb Tech 22: 261–268.
- Pras N, Hesselink PGM, Tusscher JT, Malingre Th M (1989): Kinetic aspects of bioconversion of L-tyrosine in to L-DOPA by cells of Mucuna pruriens. L. entrapped in different matrices. Biotechnol Bioeng 34: 214–222.
- Siddhuraju P, Becker K (2001): Rapid reversed-phase high performance liquid chromatographic method for the quantification of L-DOPA (L-3,4-dihydroxy phenyl alanine), nonmethylated and methylated tetrahydroisoquinoline compounds from Mucuna. beans. Food Chem 72: 389–394.
- Steiner U, Schliemann W, Strack D (1996): Assay for tyrosine hydration activity of tyrosinase from betalain-forming plants and cell cultures. Anal Biochem 238: 72–75.
- Vilanova E, Manjon A, Iborra JL (1984): Tyrosine hydroxylase activity of immobilized tyrosinase on enzacryl-AA and CPG-AA supports: Stabilization and properties. Biotechnol Bioeng 26: 1306–1312.
- Wichers HJ, Visser JF, Huizing HJ, Pras N (1993): Occurrence of L-DOPA and dopamine in plants and cell cultures of Mucuna pruriens. and effects of 2,4-D and NaCl on these compounds. Plant Cell Tissue Organ Cult 33: 259–264.
- Wichers HJ, Wijnsma R, Visser JF, Malingre Th M, Huizing HJ (1985): Production of L-DOPA by cell suspension cultures of Mucuna pruriens.. II Effect of environmental parameters on the production of L-DOPA. Plant Cell Tissue Organ Cult 4: 75–82.