Abstract
The effect of the hydroalcoholic extract of fruits of Myristica fragrans. Houtt. (Myristicaceae) was investigated on chlorpromazine-induced glucose and triglyceride elevations in male Swiss albino mice. After 7 days of oral administration, the extract, at doses of 150 and 450 mg/kg, ameliorated the metabolic abnormalities caused by chlorpromazine as evidenced by significant reduction of glucose and triglyceride (TG) levels (maximal effect of 41% and 53% reduction of glucose and TG, respectively, at 450 mg dose, p < 0.01). The standard antidiabetic rosiglitazone at 10 mg significantly (p < 0.01) reduced the TG (63%) and glucose (40%) levels in this model, while the standard antidiabetic glimepiride has exhibited 55% and 16% reduction in TG and glucose, respectively. In rats fed a high-cholesterol diet, Myristica fragrans. extract significantly reduced the elevated TG (47% reduction at 450 mg, p < 0.01) and cholesterol (66.7% reduction at 450 mg, p < 0.01), and also exhibited a reduction in hepatic TG secretion after tyloxapol administration. These data suggest that Myristica fragrans. extract ameliorates hyperglycemia and abnormal lipid metabolism in animal models.
Introduction
Type 2 diabetes mellitus (T2DM) is a major and growing health problem throughout the world. T2DM results from both peripheral insulin resistance and impaired insulin secretion. Insulin resistance arises as a consequence of obesity, a sedentary lifestyle, and aging, with resulting hyperglycemia and diabetes, blood pressure elevation, and dyslipidemia, collectively called “metabolic syndrome X” (Arulmozhi & Portha, Citation2006). A worldwide epidemic of obesity, insulin resistance, and diabetes is one of the major risk factors responsible for the ever-increasing incidence of heart failure. Evidence from human and animal studies suggests that lipid accumulation in the heart, skeletal muscle, pancreas, liver, and kidney plays an important role in the pathogenesis of heart failure, obesity, and diabetes. Cardiac lipid accumulation induces lipototoxicity, predisposing the myocytes to death and contractile dysfunction (Zhou et al., Citation2000; Chiu et al., Citation2001).
Currently available therapies for diabetes include insulin and various oral antidiabetic agents, such as sulfonylureas, metformin, α-glucosidase inhibitors, and the thiazolidinedione class of glitazones. Each of these oral agents can cause a number of serious side effects (Zang & Moller, Citation2000). Hence, there is an imperative need for better therapeutic agents with fewer side effects for the pharmacotherapy of type 2 diabetes. Plants have provided usable sources of drugs, and many drugs are directly or indirectly derived from plants. Phytomedicines have three distinct advantages: (1) Botanical extracts can be directly evaluated without initial chemical isolation for clinical efficacy, (2) increased solubility/bioavailability in crude forms, and (3) synergy between different active constituents. The biologically active components of plants useful in the treatment of diabetes include flavanoids, alkaloids, glycosides, polysaccharides, and peptidoglycans (Grover et al., Citation2002).
Myristica fragrans. Houtt. (Myristicaceae) (MF) is an evergreen aromatic tree commonly known as nutmeg or mace, and has been used traditionally as a spice and for medicinal purposes as carminative, digestive, and expectorant in the Indian system of medicine. In the recent literature, MF has been investigated for its hypolipidemic, antithrombotic, antiplatelet aggregating, antifungal, aphrodisiac, anxiogenic, anti-ulcerogenic, antitumor, and anti-inflammatory activities (Ozaki et al., Citation1989; Park et al., Citation1998; Capasso et al., Citation2000; Sonavane et al., Citation2001Citation2002; Morita et al., Citation2003; Chung et al., Citation2006). MF has been reported to contain 25–30% fixed oils and 5–15% volatile oils such as camphene, elemicin, eugenol, isoelemicin, isoeugenol, methoxyeugenol, pinen, sabinene, safrol, and also chemical substances such as dihydroguaiaretic acid, myristic acid, myristicin, and ligan (Forrest & Heacock, Citation1972; Isogai et al., Citation1973; Janssen et al., Citation1990).
In some preliminary studies, MF was found to have a hypolipidemic effect in rabbits (Sharma et al., Citation1995; Ram et al., Citation1996). However, no reports of detailed investigations of MF are available on the antidiabetic and antihyperlipidemic effects. Hence, the objectives of the current study are to investigate the antidiabetic effect of MF in mice and its antihyperlipidemic effect in high-cholesterol-fed rats.
Materials and Methods
Plant material and extraction procedure
Pharmacognostically identified fruits of Myristica fragrans. Houtt. (Myristicaceae) were collected from the local market and authenticated by Dr. A.M. Mujumdar, taxonomist, Agharkar Research Institute (Pune, India). A voucher specimen (no. M5621) was deposited in the herbarium of the institute. The hydroalcoholic extract was prepared as follows: dried and powdered rhizome (100 g) was soaked in 400 mL of 50% ethanol for 16 h at room temperature. The percolate was then decanted, centrifuged, and filtered through Whatman (no. 1) filter paper to obtain a clear extract. The extracted plant material was again subjected to the same extraction procedure. The percolates were pooled, concentrated, and lyophilized (15–18% yield).
Chemicals
Chlorpromazine (CPZ), cholesterol, sodium cholate, tyloxapol, and fenofibrate were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Rosiglitazone and glimepiride were gifts from Sun Pharma (Vadodara, India) and Torrent Pharmaceuticals (Ahmedabad, India), respectively. All the drugs were solubilized in 0.3% Tween 80 and administered orally between 1000 and 1,100 h for 7 days. All other chemicals and reagents were of pure analytical grade obtained from local suppliers.
Animals
Adult male Swiss albino mice (22–26 g) (six animals per group per treatment) and male Wistar rats (four to six animals per group per treatment) were obtained from the National Toxicology Center, Pune, India. On arrival, the animals were placed at random and allocated to treatment groups in polypropylene cages with paddy husk as bedding. Animals were housed at a temperature of 24 ± 2°C and relative humidity of 30–70%. A 12:12 light:dark cycle was followed. All animals had free access to water and standard pellet laboratory animal diet. All the experimental procedures and protocols used in this study were reviewed and approved by the Institutional Animal Care and Use Committee of Poona College of Pharmacy, Pune, India.
Effect of MF on chlorpromazine-induced hyperglycemia and hypertriglyceridemia
Male Swiss albino mice were used at 8 weeks of age. Mice were administered chlorpromazine (10 mg/kg, p.o.), concomitantly with either MF (50, 150, or 450 mg/kg, p.o.) or glimepiride (10 mg/kg, p.o.) or rosiglitazone (10 mg/kg, p.o.) or fenofibrate (100 mg/kg, p.o.) for 7 days. Animals in the control group received vehicle (0.3% Tween 80 in water, 10 ml/kg, p.o.). Blood samples were collected in fed state from the animals under mild ether anesthesia from retro-orbital sinus 1 h after drug administration.
Effect of MF on hyperlipidemic rats
Male Wistar rats were made hyperlipidemic by feeding a high-fat diet containing 2% cholesterol and 1% sodium cholate mixed with standard laboratory chow (Chakrabarti et al., Citation2004) and treated orally with MF (50, 150, or 450 mg/kg) or fenofibrate for 7 days. Effect of MF on hepatic triglyceride output was measured by intraperitoneal injection of tyloxapol at 300 mg/kg (5 mL/kg in saline). Plasma triglyceride levels were measured at 0, 4, 6, and 24 h after injection.
Determination of plasma metabolic parameters
Plasma obtained from the mice and rats was used to estimate the metabolic parameters. Glucose, triglyceride, and total cholesterol levels were measured spectrophotometrically using commercially available kits (Bayer Diagnostics, Vadodara, India).
Statistical analysis
Values are expressed as mean values ± SEM. The statistical significance of differences between the mean values was analyzed by ANOVA and Dunnett's test. A p value of < 0.05 was considered to be significant.
Results
Effect of MF on chlorpromazine-induced hyperglycemia and hypertriglyceridemia
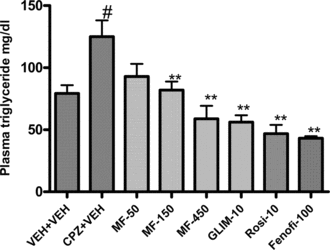
Administration of chlorpromazine significantly (p < 0.05) elevated the plasma triglyceride and glucose levels in Swiss albino mice. The triglyceride (TG) values were found to be 79.33 ± 6.55 mg/dL in vehicle-treated animals and 125.00 ± 13.15 mg/dL in chlorpromazine-treated animals. Treatment with MF dose-dependently reduced the elevated TG levels in chlorpromazine (CPZ)-treated animals and at doses of 150 mg and 450 mg, the effect (81.88 ± 6.93 and 58.78 ± 10.56, respectively) was statistically significant (p < 0.01) and comparable with that of the standard drugs glimepiride, rosiglitazone, and fenofibrate.
Treatment of MF also significantly reduced the elevated glucose levels in CPZ-treated animals. At doses of 150 and 450 mg, the effect was significantly different from the vehicle-treated animals (246.57 ± 26.17 mg/dL in vehicle vs. 175.00 ± 6.28 and 143.72 ± 6.16 for 150 mg and 450 mg, respectively). Interestingly, the glucose values were significantly reduced only by rosiglitazone, where glimepiride and fenofibrate had little and no effect, respectively.
Effect of MF on hyperlipidemic rats
In high-cholesterol-fed rats, MF exhibited dose-dependent reduction of triglyceride and cholesterol levels with respect to vehicle-treated animals. A significant (p < 0.01) maximum reduction of 47% at 450 mg/kg, on TG levels, while 66.7% reduction (p < 0.01) was observed for cholesterol with the same dose. The positive control, fenofibrate, also significantly (p < 0.01) reduced TG and cholesterol levels at 100 mg/kg ().
Table 1.. Effect of MF on metabolic parameters in high-cholesterol-fed rats.
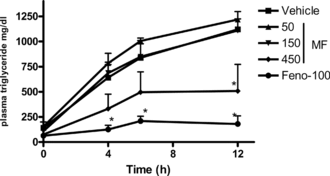
The low and intermediate doses of MF (50 and 150 mg/kg) did not produce any significant effect on hepatic triglyceride secretion in high-cholesterol-fed animals, though a mild reduction in TG levels was observed than in the vehicle animals. However, at 450 mg/kg, MF significantly (p < 0.05) reduced the TG levels at 24 h after tyloxapol administration. The positive control, fenofibrate, also reduced the TG levels at various time points ().
Figure 3 Effect of Myristica fragrans. on hepatic triglyceride secretion after the administration of tyloxapol in high cholesterol diet fed rats. Compounds were administered orally for 7 days. Each point represents mean ± SEM from n = 4 to 6. *p. < 0.005 and **p < 0.001 compared with vehicle treatment. MF, Myristica fragrans.; Feno, fenofibrate.
Discussion
To our knowledge, there is no previous ethnomedical evidence that extracts of Myrsitica fragrans. would be useful in the treatment of hyperglycemia. Though reports on hypolipidemic effects are available, only a single dose of MF has been employed in rabbits (Sharma et al., Citation1995; Ram et al., Citation1996). Our decision to explore this possibility was based on the observation that terpenoid-type quinines isolated from the tree Pycnanthus angolensis. Welw (Myristicaceae), also known as African nutmeg, have marked antidiabetic effects in mouse models of type 2 diabetes (Luo et al., Citation1999).
Based on this observation, we have evaluated the ability of crude extract of MF to lower plasma glucose and TG concentrations in chlorpromazine-induced type 2 diabetes in mice. This effort was successful, as attested to by the results shown in and , documenting the fact that MF is capable of significantly lowering plasma glucose concentrations when given orally to a mouse model of type 2 diabetes.
Figure 1 Effect of Myristica fragrans. on chlorpromazine-induced elevations of plasma glucose levels in mice. Compounds were administered orally for 7 days. Bars represent means ± SEM from n = 6. *p. < 0.005 and **p < 0.001 compared with vehicle treatment. MF, Myristica fragrans.; Fenofib, fenofibrate; Rosi, rosiglitazone; CPZ, chlorpromazine; Glim, glimepiride. #p. < 0.05 compared to vehicle/vehicle treated animals.
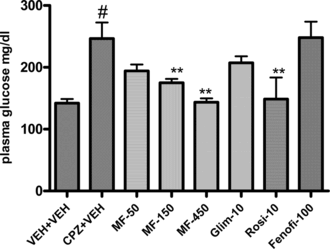
Figure 2 Effect of Myristica fragrans. on chlorpromazine-induced elevations of plasma triglyceride levels in mice. Compounds were administered orally for 7 days. Bars represent means ± SEM from n = 6. *p. < 0.005 and **p < 0.001 compared with vehicle treatment. MF, Myristica fragrans.; Fenofib, fenofibrate; Rosi, rosiglitazone; CPZ, chlorpromazine; Glim, glimepiride. #p. < 0.05 compared to vehicle/vehicle treated animals.
Three major classes of oral antidiabetic agents that are in use today act through three distinct mechanisms:sulfonylureas by inducing insulin secretion, acarbose by inhibiting carbohydrate absorption, and thiazolidinediones by insulin sensitization.
It has been reported that chlorpromazine-induces hyperglycemia in mice (Dwyer & Donohoe, Citation2003). The antagonism at muscarinic acetylcholine receptors is suggested as one of the mechanism for the metabolic abnormalities with chlorpromazine (Xie & Lautt, Citation1995). MF has recently been reported to possess procholinergic activity (Parle et al., Citation2004) and significantly reduced the carbachol-induced gastric acid secretion in preclinical models (Jan et al., Citation2005). These results suggest that the procholinergic effect may slow the digestion of food and decrease the carbohydrate absorption. In the current study, MF significantly reduced the plasma glucose levels after 7 days of treatment. The effect observed at 450 mg/kg dose was comparable with that of known thizolidinedione rosiglitazone (10 mg/kg). Also, the elevated TG levels observed after the administration of chlorpromazine was significantly lowered with MF treatment. Because the chlorpromazine-induced metabolic abnormalities (elevated triglycerides and glucose) have not been studied for the evaluation of antidiabetic compounds, we have employed glimepiride (a sulfonylurea with extrapancreatic effects), rosiglitazone, a thizolidinedione, and fenofibrate, a known lipid-lowering agent, to validate the current model. As expected, all the three agents significantly reduced the plasma TG levels after 7 days of treatment, and fenofibrate did not exhibit any effect on plasma glucose levels.
Male Wistar rats, when fed with a high-cholesterol-containing diet, showed a significant increase in plasma total and LDL-cholesterol and also plasma triglyceride levels with a concomitant decrease in HDL-cholesterol levels. No change in plasma glucose level was observed. When treated with MF, these animals showed a dose-dependent improvement in plasma lipid levels. The high cholesterol diet fed rat model has been used previously to study the efficacy of fibrates (Petit et al., Citation1988; Chakrabarti et al., Citation2004). These animals are hypercholesterolemic, hypertriglyceridemic, but are nondiabetic. Normally, rodent plasma total cholesterol contains a very high proportion of HDL-cholesterol and very low LDL-cholesterol. This makes therapeutic interpretation of cholesterol lowering in normal rodents difficult. In the case of hyperlipidemic rats, plasma cholesterol is predominantly LDL-cholesterol, which more closely reflects the clinical situation. MF showed comparable efficacy at doses of 150 and 450 mg/kg with fenofibrate (100 mg/kg) in this model.
MF, at 450 mg/kg, significantly inhibited the lipoprotein secretion in high-cholesterol-fed rats. This assay is based on intraperitoneally injected tyloxapol to prevent the catabolism of TG-rich lipoproteins (Li et al., Citation1996). Thus, after the administration of tyloxapol, there is a linear increase in the TG levels, which reflects the TG secretion rate from the liver. It may be postulated that MF has some effect on the liver TG secretion.
Conclusions
In summary, for the first time we have demonstrated that the ethanol extract of Myristica fragrans. fruit ameliorates hyperglycemia and abnormal lipid metabolism in animal models. Further pharmacodynamic investigations in appropriate models of type 2 diabetes are required to understand the precise mechanism of antihyperglycemia and antihyperlipidemia exhibited by Myristica fragrans..
Acknowledgments
We thank Dr. S.S. Kadam and Dr. K.R. Mahadik (Bharati Vidyapeeth Deemed University, Poona College of Pharmacy, Pune) for their constant encouragement in the work.
References
- Arulmozhi DK, Portha B (2006): GLP-1 based therapy for type 2 diabetes. Eur J Pharm Sci 28: 96–108.
- Capasso R, Pinto L, Vuotto ML, Di Carlo G (2000): Preventive effect of eugenol on PAF and ethanol induced gastric mucosal damage. Fitoterapia 71: S131–S137.
- Chakrabarti R, Mishra P, Vikramadithyan RK, Premkumar M, Hiriyan J, Datla SR, Darmala RKB, Suresh J, Rajagopalan R (2004): Antidiabetic and hypolipidemic potential of DRF 2519-a dual activator of PPAR-α and PPAR-γ. Eur J Pharmacol 491: 195–206.
- Chiu HC, Kovacs A, Blanton DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE (2001): A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest 107: 813–522.
- Chung JY, Choo JH, Lee MH, Hwang JK (2006): Anticariogenic activity of macelignan isolated from Myristica fragrans. (nutmeg) against Streptococcus mutans.. Phytomedicine 13: 261–266.
- Dwyer DS, Donohoe D (2003): Induction of hyperglycemia in mice with atypical antipsychotic drugs that inhibit glucose uptake. Pharmacol Biochem Behav 75: 255–260.
- Forrest JL, Heacock RA (1972): Identification of the major components of the essential oil of mace. J Chromatogr A 69: 115–121.
- Grover GK, Yadav S, Vats V (2002): Medicinal plants in India with antidiabetic potential. J Ethnopharmacol 81: 81–100.
- Isogai A, Suzuki A, Tamura S (1973): Structure of dimeric phenoxyprapanoids from Myristica fragrans.. Agric Biol Chem 37: 193–194.
- Jan M, Faqir F, Jamida, Mughal MA (2005): Comparison of effects of extract of Myristica fragrans. and verapamil on the volume and acidity of carbachol induced gastric secretion in fasting rabbits. J Ayub Med Coll Abbottabad 17: 69–71.
- Janssen J, Laekeman GM, Peiters LA, Totte J, Herman AG, Vlietinck AJ (1990): Nutmeg oil: Identification and quantification of its most active constituents as inhibitors of platelet aggregation. J Ethnopharmacol 29: 179–188.
- Li X, Catalina F, Grundy SM, Patel S (1996): Method to measure the apolipoprotein B-48 and B-100 secretion rates in an individual mouse: Evidence for a very rapid turnover of VLDL and preferential removal of B-48 relative to B-100 containing lipoproteins. J Lipid Res 37: 210–220.
- Luo J, Cheung J, Yevich EM, Clark JP, Tsai J, Lapresca P, Ubillas RP, Fort DM, Carlson TJ, Hector RF, King SR, Mendez CD, Jolad SD, Reaven GM (1999): Novel terpenoid-type quinones isolated from Pycnanthus angolensis. of potential utility in the treatment of type 2 diabetes. J Pharmacol Exp Ther 288: 529–534.
- Morita T, Jinno K, Kawagishi H, Arimoto Y, Suganuma H, Inakuma T, Sugiyama K (2003): Hepatoprotective effect of myristicin from nutmeg (Myristica fragrans.) on lipopolysaccharide/d-galactosaminde induced liver injury. J Agri Food Chem 51: 1560–1565.
- Ozaki Y, Soedigdo S, Wattimena YR, Suganda AG (1989): Antiinflammatory effect of mace, aril of Myristica fragrans. Houtt., and its active principles. Jpn J Pharmacol 49: 155–163.
- Park S, Lee DK, Yang CH (1998): Inhibition of foc-jun-DNA complex formation by dihydroguaiaretic acid and in vitro. cytotoxic effects on cancer cells. Cancer Lett 127: 23–28.
- Parle MN, Dhingra D, Kulkarni SK (2004): Improvement of mouse memory by Myristica fragrans. seeds. J Med Food 7: 157–161.
- Petit D, Bonnefis MT, Rey C (1988): Effects of ciprofibrate and fenofibrate on liver lipids and lipoprotein synthesis in normo- and hyperlipidemic rats. Atherosclerosis 74: 215–225.
- Ram A, Luria P, Gupta R, Sharma VN (1996): Hypolipidaemic effect of Myristica fragrans. fruit extract in rabbits. J Ethnopharmacol 55: 49–53.
- Sharma A, Mathur R, Dixit VP (1995): Prevention of hypercholesterolemia and atherosclerosis in rabbits after supplementation of Myristica fragrans. seed extract. Indian J Physiol Pharmacol 4: 407–410.
- Sonavane G, Sarveiya V, Kasture V, Kasture S (2001): Behavioral actions of Myristica fragrans. seeds. Indian J Pharmacol 33: 417–424.
- Sonavane GS, Sarveiya VP, Kasture VS, Kasture SB (2002): Anxiogenic activity of Myristica fragrans. seeds. Pharmacol Biochem Behav 71: 239–244.
- Xie H, Lautt WW (1995): Induction of insulin resistance by cholinergic blockade with atropine in the cat. J Autn Pharmacol 15: 361–369.
- Zang BB, Moller DE (2000): New approaches in the treatment of type 2 diabetes. Curr Opin Chem Biol 4: 461–467.
- Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unfger RH (2000): Lipotoxic heart disease in obese rats: Implications for human obesity. Proc Natl Acad Sci, USA 97: 1784–1789.