Abstract
The immunomodulating activities of polysaccharides from Dendrobium huoshanense. C. Z. Tang et S. J. Cheng (Orchidaceae) (HPS) were assessed. Crude HPS at concentrations of 100 to 200 µg/mL significantly stimulated tumor necrosis factor-α. (TNF-α.) release in the supernatant of peritoneal macrophages, and interferon-γ. (IFN-γ.) release in the supernatant of murine splenocytes was also enhanced by 100 to 800 µg/mL of crude HPS. The maximum values of 542.9 pg/mL (TNF-α.) and 790.1 pg/mL (IFN-γ.) were obtained at the 24th hour of culture. RT-PCR analysis indicated that enhancement of both TNF-α. and IFN-γ. release by HPS was attributed to the upregulation of the expression of the corresponding genes in cells. Different HPS fractions showed different activities. Among all fractions from anion-exchange chromatography and gel filtration, three fractions of HPS1A, HPS1B, and HPS2B had more potent effect on IFN-γ. and TNF-α. release than the crude HPS. These results suggest that HPS might induce an immune response.
Introduction
Some plant polysaccharides are well-known to possess immunoregulatory effects and to exhibit significant antitumor and antiviral activities (Liu et al., Citation1998Citation1999; Qiu et al., Citation2000). Dendrobium. (Orchidaceae), a rich source of polysaccharides, has traditionally been used worldwide as a folk remedy for various diseases because of its biological activities in healing cataract, throat inflammation, peripheral obstruction, and chronic superficial gastritis (Chen & Guo, Citation2000; Hua et al., Citation2004). Previous studies had indicated that polysaccharides of Dendrobium candidum. C. Z. Tang et S. J. Cheng could enhance the killing activities of LAK cells of umbilical cord blood and peripheral blood of cancer patients in vitro. (Luo et al., Citation2000). Dendrobium huoshanense. is among the most famous traditional Chinese medicine herbs used for centuries. Polysaccharides have been extracted from the species, but biological activities have not been reported (Luo et al., Citation2003). The aim of this study is to investigate the immunopotentiating activity of polysaccharides from Dendrobium huoshanense..
Materials and Methods
Reagents
Concanavalin A (Con A), LPS, DEAE-cellulose, and Sephadex G25 were purchased from Sigma. RPMI 1640, TRIzol reagents and M-MLV reverse trancriptase were from GIBCO BRL. Taq DNA polymerase was from Promega. The gel of Sephacryl S200 was from Amacia. ELISA kits of IFN-γ. and TNF-α. were from Jingmei Biotech Co., Ltd.
Preparation of the crude polysaccharides of Dendrobium huoshanense.
The powdered material (20 g) was pre-extracted for 48 h in a Soxhlet system with acetone and subsequently for another 48 h with MeOH. The extract was discarded. The residue was dried at 40°C and extracted three-times with 100 mL hot distilled water and 0.5 g polyvinylpyrrolidone. The combined extracts were concentrated to about 60 mL and centrifuged at a speed of 12,000 × g. for 30 min. The supernatant was poured into 240 mL of 95% EtOH and centrifuged, and the precipitate was dissolved in 60 mL of distilled water. This process was repeated three-times. The precipitate was then washed with Sevag reagent (isoamyl alcohol and CHCl3 in 1:4 ratio) (Bao et al., Citation2002) and dried in a vacuum, to give the crude polysaccharide in Dendrobium huoshanense., HPS.
Fractionation and purification
Anion exchange chromatography
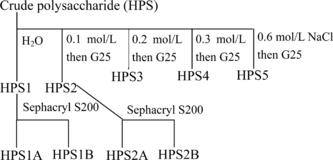
The crude polysaccharides of Dendrobium huoshanense. (HPS) was separated into five fractions of HPS1, HPS2, HPS3, HPS4, and HPS5 by elution from DEAE-cellulose anion-exchange chromatography column (20 × 2.5 cm) with a NaCl gradient (0 to 0.6 m NaCl) (). Desalting was done by gel filtration on Sephadex G25 in water.
Gel filtration
The main components, eluted in the void volume, were collected, and, after lyophilization, the obtained products (HPS1 and HPS2) were purified successfully on Sephacryl S200 (60 × 1.6 cm), with 0.1 M phosphate buffer as the eluant. Two fractions of HPS1A and HPS1B were obtained from HPS1 and two fractions of HPS2A and HPS2B from HPS2.
Animals
Male BALB/c mice (18±2 g, 8–10 weeks old) were purchased from the Experimental Animal Center, Anhui Medical University of China. The mice were housed under normal laboratory conditions (i.e., room temperature, 12/12 h light-dark cycle with free access to standard rodent chow and water).
Preparation of murine splenocytes and peritoneal macrophages
Mice were sacrificed and peritoneal macrophages were recovered by lavage of the peritoneal cavity with ice-cold Hank's balanced salt solution (HBSS), and the murine splenocytes were obtained by homogenizing spleen with ice-cold HBSS. For splenocyte preparation, the single cell suspension from the teased murine was obtained by passing it through a 200-mesh and hemolyzed by the buffer solution containing 1 mM Tris-HCl and 1% NH4Cl (pH 7.2). Cells were washed twice with RPMI 1640 medium and subsequently suspended in complete RPMI 1640 culture medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin. Cell viability was determined by Trypan blue dye exclusion, and the cell concentration was adjusted to 2.0 × 106/mL.
Cytokine bioassay
For interferon-γ. (IFN-γ.) determination, splenocytes (2 × 106 cells/mL) were treated by different concentrations of HPS in the presence of Con A (5 µg/mL). After incubation for 6, 12, 24, 48, and 72 h at 37°C in a humidified 5% CO2 incubator, the cell supernatants were collected and IFN-γ. was measured by ELISA kits according to the recommendation of the manufacturer. As described above, where only Con A was replaced by LPS (10 µg/mL), the supernatant of peritoneal macrophages was also collected for tumor necrosis factor-α. (TNF-α.) determination by ELISA kits.
RT-PCR for cytokine gene expression
Splenocytes (treated with 800 µg/mL HPS for 6, 12, 24, 48, and 72 h in the presence of 5 µg/mL Con A) and peritoneal macrophages (treated with 200 µg/mL HPS for 6, 12, 24, 48, and 72 h in the presence of 10 µg/mL LPS) were washed and the RNA was extracted with TRIzol reagent according to the recommendation of the manufacturer. RNA concentration was determined by measuring the optical density at 260 nm. RT-PCR was carried out according to Liu with some modifications (Liu et al., Citation1998).
RNA was reverse-transcribed at 37°C for 1 h in a 20 µL reaction mixture after heating at 65°C for 5 min. The reverse mixture was the same as that described by Liu et al. (Citation1998). RT samples were stored at − 80°C until subjected to PCR amplification.
All PCR primer pairs specific for TNF-α., IFN-γ., and β.-actin were synthesized by Shanghai Biotechnology Company of China. The primer sequences and expected size of the PCR products are shown in (Han et al., Citation1995; Nicolas et al., Citation1997). β.-Actin (478 bp) was used as standard in this study.
Table 1.. Sequences of the primers used in RT-PCR.
The following components were added to the RT sample to make up 25µL reaction mix containing 2 µL cDNA, 2.5 µL Taq DNA polymerase buffer (100 mM Tris-HCl, pH 8.3, 500 mM KCl, 15 mM MgCl2, 1 mg/mL bovine serum albumin), 2.0 µL 2.5 mM mixture of all dNTPs, 1 µL each of 5′ and 3′ primer (60 pmol/µL), and 1 U Taq DNA polymerase. PCR was performed for 30 cycles using a DNA thermal cycler (PE 2400), each cycle consisting of 1 min of denaturation at 94°C, 1 min of annealing at 56°C, and 1 min of extension at 72°C. The reaction products were visualized by electrophoresis in 1.2% agarose gel containing 0.5 µg/mL ethidium bromide. λDNA/EcoRI + Hind\SHcy. were run in parallel as the molecular weight marker.
Statistical analysis
All experiments were carried out in a completely randomized design with three replicates per treatment. The data were analyzed statistically using one-way analysis of variance (ANOVA), and the data means were compared using Duncan's multiple range tests with the level of significance set at 5%.
Results and Discussion
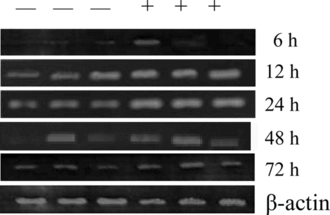
Macrophages are the antigen-presenting cells known to be both cytotoxic and phagocytic to invading microorganisms. Stimulation of macrophages enhances these functions and might therefore be a target for therapeutic applications. TNF-α., one of the cytokines released by macrophages, plays a critical role in mediating the signal transduction stimulating the immune defense system (Liu et al., Citation1998). It has been suggested that polysaccharides of some plants can stimulate the immune system (Liu et al., Citation1999; Bao et al., Citation2002). We hypothesized that polysaccharides of Dendrobium huoshanense. might also stimulate macrophage to release TNF-α. thereby stimulating the immune system. To test this hypothesis, HPS at different concentrations was added into the medium of mouse peritoneal macrophages to determine its stimulating effect on TNF-α. release from cells. There was no observed toxicity effect of the samples on cell viability at the experimental concentration range as determined by Trypan blue exclusion. As shown in , TNF-α. release by peritoneal macrophages increased when HPS concentration ranged from 100 to 200 µg/mL and then decreased at HPS concentrations of 400 and 800 µg/mL. At the concentration of 200 µg/mL, HPS stimulated TNF-α. release of 542.9 pg/mL at the 24th hour of culture, which was 1.5-fold that of control treated only with LPS. In all cases, the time-dependent influence profiles of TNF-α. release was similar. TNF-α. release was started after inoculum and reached the peak at the 24th hour of incubation followed by a decrease, which was very consistent with the expression of TNF-α. gene in peritoneal macrophages (). In the 200 µg/mL HPS and LPS (10 µg/mL) treated macrophages, the expression level of TNF-α. started to increase at the 6th hour and then dropped at the 24th hour (). This result indicated that the increase in production of TNF-α. was attributed to upregulating of TNF-α. expression in macrophages. TNF-α. is known to have a wide range of biological activities on many different target tissues, including cytotoxic T lymphocyte and macrophages (Lanni et al., Citation1997; Thomson, Citation1992; Malorni et al., Citation1996). Hence, increased production of TNF-α. results in augmented maturation capable of inducing various other cytokines such as IL-1, IL-6, and TNF-α. itself (Lanni et al., Citation1997; Thomson, Citation1992; Malorni et al., Citation1996). Furthermore, TNF-α. can also induce apoptosis of some tumor cells in vitro. (Geng & Hellstrand, Citation1996).
Figure 2 Effects of the crude polysaccharides (HPS) from Dendrobium huoshanense. on the production of TNF-α. from peritoneal macrophages induced by LPS.
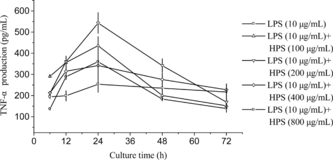
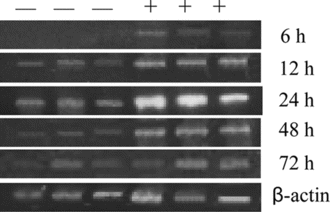
Figure 3 mRNA level of TNF-α. in peritoneal macrophages treated with 200 µg/mL crude HPS in the presence of 10 µg/mLLPS. —, mRNA level in cells treated only with LPS; +, mRNA level in cells treated with crude HPS in the presence of LPS. The three − and + represent that the two experiments were done in triplicate, respectively. β.-Actin expression at different intervals is almost the same as that shown in the figure.
Lymphocytes are also important immune cells and play a privotal role in immune responses. These cells are able to produce many kinds of cytokines after differentiation and activation. As the first step for T-cell activation, lymphocytes should be activated by various signals such as LPS or plant products including polysaccharides (Bao et al., Citation2002; Cho et al., Citation2002). The immune response can be broadly categorized into cellular- or humoral-mediated response. The two types of immune responses are separately regulated by cytokines that control two general subsets of helper cells known as Th1 and Th2. IFN-γ. is evaluated as representative Th1 cytokines mainly secreted from Th1 cells (Hsieh et al., Citation1993). Therefore, in this study, IFN-γ. was determined in splenocyte culture medium and the highest value was found in that treated with 800 µg/mL HPS in the presence of 5 µg/mL Con A at the 24th hour of culture, which was 2.5-fold higher than that of the control treated only with 5 µg/mL Con A (). On account of time-dependent influence, for all doses of HPS, IFN-γ. was released in the first 24 h followed by a decrease of different degrees. IFN-γ. release was also increased with higher doses of HPS, and the dose-dependent influence was strongly significant (p < 0.05), compared with that in cells treated only with 5 µg/mL Con A. By investigation of IFN-γ. gene expression in splenocytes treated with 800 µg/mL HPS in the presence of 5 µg/mL Con A at different intervals, it was upregulated from 6 h onwards and dramatically upregulated at 24 h followed by a drop toward the end of study (). This is in line with the profiles of IFN-γ. accumulation in the culture medium, which indicated that IFN-γ. release is also regulated by the expression of IFN-γ. gene. Similar results were also reported by Liu et al. (Citation1999). The observation that IFN-γ. plays a critical role in tumor rejection stimulated a search for the physiologically relevant cellular targets of IFN-γ. action (Dighe et al., Citation1994). Furthermore, IFN-γ. has long been recognized as a major macrophage-activating factor capable of inducing macrophages to nonspecifically kill a variety of tumor targets (Schreiber et al., Citation1986; MacMicking et al., Citation1997).
Figure 4 Effects of the crude polysaccharides from Dendrobium huoshanense. on the production of IFN-γ. from murine splenocytes induced by Con A.
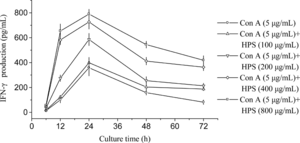
Figure 5 mRNA level of IFN-γ. in murine splenocytes treated with 800 µg/mL crude HPS in the presence of 5 µg/mL Con A. —, mRNA level in cells treated only with Con A; +, mRNA level in cells treated with the crude HPS in the presence of Con A. The three − and + represent that the two experiments were done in triplicate, respectively. β.-Actin expression at different intervals is almost the same as that shown in the figure.
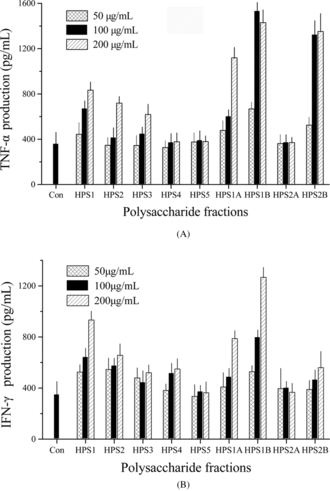
The immunoregulatory activity of the polysaccharide fractions obtained after DEAE-cellulose and gel filtration was determined on cultures of murine splenocytes and macrophage by measuring TNF-α. and IFN-γ. release. Compared with the crude polysaccharides, the purified fractions of HPS1A, HPS1B, and HPS2B had a more potent effect on IFN-γ. and TNF-α. release and it did not require high concentrations. The response of the two cytokines to HPS5 was not notable (). These results suggested that interaction on immune cells existed among the different polysaccharide fractions. Similar results were also found with other plant polysaccharides (Bao et al., Citation2002).
Figure 6 Effects of the fractions of polysaccharide from Dendrobium huoshanense. on the production of IFN-γ. (A) and TNF-α. (B).
The current study demonstrated that polysaccharides from Dendrobium huoshanense. can enhance both TNF-α. release from peritoneal macrophages and IFN-γ. release from murine spleen cells by upregulation of gene expression, which suggested that, at least partly, HPS might possess immunomodulatory activities.
Acknowledgment
This work was partially supported by the Key Project for Science and Technology Research from Ministry of Education of China (no. 03098).
References
- Bao XF, Wang Z, Fang JN, Li XY (2002): Structural features of an immunostimulating and antioxidant acidic polysaccharide from the seeds of Cuscuta chinensis.. Planta Med 68: 237–243.
- Chen XM, Guo SX (2000): Advances in the research of constituents and pharmacology of Dendrobium.. Nat Prod Res Dev 13: 70–75.
- Cho JY, Kim AR, Yoo ES, Baik KU, Park MH (2002): Ginsenosides from Panax ginseng. differentially regulate lymphocyte proliferation. Planta Med 68: 497–500.
- Coughlin CM, Salhany KE, Gee MS, La TDC, Kotenko S, Ma XL, Gri G, Wysocka M, Kim JE, Liu L, Liao F, Farber JM, Pestka S, Trinchieri G, Lee WMF (1998): Tumor cell responses to IFN-γ. affect tumorigenicity and response to IL-12 therapy and anti-angiogenesis. Immunity 9: 25–34.
- Dighe AS, Richards E, Old LJ, Schreiber RD (1994): Enhanced in vivo. growth and resistance to rejection of tumor cells expressing dominant negative IFN-γ. receptors. Immunity 1: 447–456.
- Geng YJ, Hellstrand K (1996): Apoptotic death of human leukemic cells induced by vascular cells expressing nitric oxide syntheses in response to γ.-interferon and tumor necrosis factor-α.. Cancer Res 56: 866–874.
- Han CW, Imamura M, Hashino S (1995): Differential effects of the immunosuppressants cyclosporine A, FK506 and KM2210 on cytokine gene expression. Bone Marrow Transpl 15: 733–739.
- Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM (1993): Development of TH1 CD4 + T cells through IL-12 produced by Listeria-induced macrophages. Science 260: 547–549.
- Hua YF, Zhang M, Fu CX, Chen ZH, Chan GYS (2004): Structural characterization of a 2-O.-acetylglucomannan from Dendrobium officinale. stem. Carbohydr Res 339: 2219–2224.
- Lanni JS, Lowe SW, Licitra EJ, Liu JO, Jacks T (1997): p53-Independent apoptosis induced by paclitaxel through an indirect mechanism. Proc Natl Acad Sci USA 94: 9679–9683.
- Liu F, Ooi VEC, Fung MC (1999): Analysis of immunomodulating cytokine mRNA in the mouse induced by mushroom polysaccharides. Life Sci 64: 1005–1011.
- Liu MQ, Li JZ, Kong FZ, Lin JY, Gao Y (1998): Induction of immunomodulating cytokines by a new polysaccharide-peptide complex from culture mycelia of Lentinus edodes.. Int Immunopharmacol 40: 187–198.
- Luo HL, Cai TY, Chen QL, Huang MQ, Mei CG, Li YQ (2000): Enhancement of Dendrobium candidum. polysaccharide on killing effect of LAK cells of umbilical cord blood and peripharal blood of cancer patients in vitro.. Cancer ( Chinese) 19: 1124–1126.
- Luo JP, Zha XQ, Jiang ST (2003): Suspension culture of protocorm-like bodies from the endangered medicinal plant Dendrobium huoshanenese.. Chin J Chinese Mater Med 28: 20–23.
- MacMicking J, Xie QW, Nathan C (1997). Nitric oxide and macrophage function. Annu Rev Immunol 15: 323–350.
- Malorni W, Rainaldi G, Tritarelli E, Rivavbene R, Cianfriglia M, Lehnert M, Donelli G, Peschele C, Testa U (1996): Tumor necrosis factor α. is a powerful apoptotic induce in lymphoid leukemic cells expression the P-170 glucoprotein. Int J Cancer 67: 238–247.
- Nicolas F, Gerard B, Werner R (1997): Comparison of cytokine measurements using ELISA, ELISPOT and semi-quantitative RT-PCR. J Immunol Methods 204: 57–66.
- Qiu ZH, Ken J, Mark W, Jia Q, Orndorff S (2000): Modified Aloe barbadensis. polysaccharide with immunoregulatory activity. Planta Med 66: 152–156.
- Schreiber RD, Celada A, Buchmeier N (1986): The role of interferon-γ. in the induction of activated macrophages. Ann Inst Pasteur Immunol 137C: 203–206.
- Thomson A (1992): The Cytokine Handbook, Third printing. San Diego, Academic Press, pp. 241–256.