Abstract
Five species of lichens, Ramalina fastigiata. (Pers.) Ach., Ramalina capitata., (Ach.) Nyl., Ramaliana polymorpha. (Lilj.) Ach., Ramalina pollinaria. (Westr.) Ach., and Ramalina fraxinea. (L.) Ach., belonging to family Ramalinacae. were collected from middle Anatolia, Ankara, Antalya, Karabük, and Kayseri in Turkey. Their usnic acid amounts were determined by HPLC in acetone extracts. Furthermore, antimicrobial activities of these extracts were determined against Escherichia coli. (ATCC 35218), Enterococcus faecalis. (RSKK 508), Proteus mirabilis. (Pasteur Ens. 235), Staphylococcus aureus., Bacillus subtilis., and Bacillus megaterium.. It was observed that, as the usnic acid amount increased, the antimicrobial activities were increased. Results pointed out that usnic acid contents of Ramalina. species varied between 0.13% and 3.23% dry weight.
Keywords:
Introduction
Lichens are formed through symbiosis between fungi and algae and/or cyanobacteria. Lichens have been used in folk medicine in many countries over a considerable period of time. Their efficacy is due to the synthesis of unique secondary compounds, a number of which have important biological roles as antibiotic, antiherbivore, and antimicrobial compounds (Lawrey, Citation1989). Although these manifold activities of lichen metabolites have now been recognized, their therapeutic potential has not yet been fully explored and, thus, remains pharmaceutically unexploited. Recently, aliphatic acids, pulvinic acid derivatives, depsides and depsidones, dibenzofuans, anthraquinones, naphthoquinones, as well as epidithiopiperazinediones were described. An improved access to these lichen substances in drug discovery high-throughput screening programs will provide impetus for identifying novel lead-compounds with therapeutic potential and poses new challenges for medicinal chemistry (Müller, Citation2001).
A great number of lichen species have proved to be a source of important secondary metabolites for the food and pharmaceutical industries. Although many natural lichens and cultured lichens have been screened for their biological activities and several novel compounds have been isolated and identified, lichens have been essentially ignored by the modern pharmaceutical industry because of their slow growth in nature. Industrial-scale harvests are neither ecologically sensible nor sustainable and, for many species, are not feasible (Crittenden & Porter, Citation1991; Lauterwerwein et al., Citation1995; Yamamoto et al., Citation1998; Miao et al., Citation2001; Behera et al., Citation2003). In particular, Ramalina. species were most commonly used for medicinal, perfumery, and cosmetics. Usnic acid as a pure substance has been formulated in creams, toothpaste, mouthwash, deodorants, and sunscreen products, in some cases as an active principle, in others as a preservative.
The antibiotic mechanism of many lichens results from usnic acid. The antimicrobial agent usnic acid has activity against Gram-negative bacteria and mycobacteria. In addition to antimicrobial activity against human and plant pathogens, usnic acid has been reported to exhibit antiprotozoal, antiproliferative, anti-inflammatory, analgesic, and antiviral activity (Ingolfsdottir, Citation2002).
In this study, usnic acid contents of five Ramalina. species belonging to family Ramalinacae. (Ramalina fastigiata. (Pers.) Ach., Ramalina capitata. (Ach.) Nyl., Ramalina polymorpha. (Lilj.) Ach., Ramalina pollinaria. (Westr.) Ach., and Ramalina fraxinea. (L.) Ach.) collected from different regions of Turkey (Ankara, Antalya, Karabük, Kayseri) were detected. Besides this, the study constitutes a contribution to the determination of usnic acid content in some lichens from Turkey. In Turkey, despite widespread belief in the pharmaceutical and bioactive characteristics of lichens, there is little scientific evidence to support its use. Usnic acid is thought to be responsible for its beneficial actions in various diseases. This work attempts to contribute to increase knowledge about the antimicrobial effects of the lichens from Turkey. The objective of the current study on the lichens was to determine the antimicrobial activity, which has not been previously evaluated.
Materials and Methods
Lichen materials
The lichens collected were Ramalina capitata., Kayseri Erciyes Mountain; north of Perikartιn in July 2002 (northern slope of Erciyes Mountain), 38° 35′N, 35° 27′E, 2300 m; Ramalina polymorpha., Ankara-Beynam Forest in July 2003, 36°53′920″N, 32°55′005″E, 1381 m; Ramalina fraxinea., Kayseri Erciyes Mountain, north of Perikartιn in July 2002 (northern slope of Erciyes Mountain), 38°35′N, 35°27′E, 2300 m; Ramalina pollinaria., Karabük Yaylacιk Forest, Kaplιkaya Hill, in June 2005, 41°06′N, 32°18′E, 500 m; and Ramalina fastigiata., Antalya Termosos Natural Park, Güllük Mountain, in May 2002, 37°58′N, 30°31′E 830 m. These Ramalina. species were identified by Demet Cansaran (Ramalina polymorpha., Ramalina pollinaria., Ramalina fastigiata.) and M. Gökhan Halιcι (Ramalina capitata., Ramalina fraxinea.). The samples were dried at room temperature and foreign matter was removed prior to grinding. The lichen samples are stored in the herbarium of Erciyes University (Erciyes University, Department of Botany, Kayseri, Turkey) and Ankara University-ANK (Ankara University, Deparment of Botany, Ankara, Turkey).
Determination of antimicrobial activity
Test microorganisms
The test microorganisms Escherichia coli. (ATCC 35218), Enterococcus faecalis. (RSKK 508), Proteus mirabilis. (Pasteur Ens. 235), Staphylococcus aureus., Bacillus subtilis., Bacillus megaterium., and Pseudomonas aeruginosa. were obtained from Refik Saydam National Type Culture Collection (RSSK) and Ankara University, Faculty of Science, Department of Biology.
Preparation of lichen extracts for antimicrobial activity
From dried lichen samples, 0.05 g were weighed and put into screw-capped glass tubes. Extraction was performed by adding 10 mL of acetone with 1 h incubation at room temperature. Chemicals used for extraction were obtained from Sigma (Germany) and were of the highest grade available. At the end of the incubation period, tubes were centrifuged to remove lichens from supernatants. These extracts were used in the experiments.
Antimicrobial activity assays
For screening of antimicrobial activity, the agar disk diffusion method was used. The extracts (50 µL) were dried on 6-mm filter paper disks. In addition, control disks were prepared with solvents free of lichen extract in order to determine the antimicrobial activity of solvent acetone. Tetracycline (30 µg/disk) was used as reference. For antimicrobial assays, all bacterial strains were grown in nutrient broth medium (Oxoid, UK) for 24 h at 37°C. Then, 0.1 mL of each culture of bacteria was spread on nutrient agar plate surfaces. After that, disks were placed onto agar Petri plates and incubated. The inhibitory activity was indicated by clear zones around the disks, and inhibition zone diameters were measured in millimeters after incubation for 24 h at 37°C (Perry et al., Citation1999). All tests were performed in triplicate.
Determination of HPLC analysis of the lichen samples
Sample preparation for HPLC analysis
Air-dried lichens were ground and extracted with 0.05 g amount in 10 mL acetone at room temperature (20–22°C). The extracts were stored in darkness at 4°C until HPLC analysis. Before analysis, extracts were passed through 0.45-µm filters and then injected into the HPLC system in amounts of 20 µL.
Standard and solvents
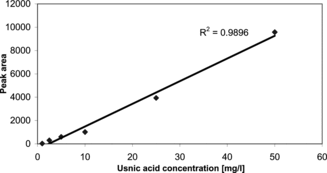
All of the chemicals used in experiments were of HPLC grade from Sigma and of highest. A stock solution of 1 mg/mL usnic acid was prepared in acetone. An appropriate dilution of this stock solution was made with acetone. All of the standards were placed in an autosampler and analyzed. Calibration curves for usnic acid were obtained with seven samples of various concentrations using linear regression analysis ().
Analytical conditions and apparatus
A Thermo Finnigan HPLC System (USA) equipped with a Surveyor LC pump, Surveyor photodiode array detector, Surveyor autosampler and data processor (ChromQuest 4.01) was used. Reverse-phase Shim-pack CLC-ODS (M), 5-µm particle size, in a 250 mm × 4.6 mm i.d. stainless steel column was used. Flow rate was 0.8 mL/min. For usnic acid detection at 245 nm, a methanol phosphate buffer (pH 7.4) (70:30 v/v) was used as a mobile phase (Feige et al., Citation1993). Aliquots of the extracts, (20 µL) were injected into the HPLC system. Each analysis was carried out in triplicate.
Results
In this study we tested the antimicrobial activity of the acetone extract of Ramalina capitata., R. polymorpha., R. fraxinea., R. pollinaria., and R. fastigiata. against seven test bacteria. The study showed that lichen extracts have antimicrobial effects against the tested bacteria at different rates. Results from antimicrobial activity tests are given in .
Table 1.. Antimicrobial activity of various lichen extracts.
The acetone extracts of Ramalina fastigiata. and Ramalina capitata. were found to be effective on most of the tested bacteria. The extract of Ramalina fastigiata., which is the most efficient, showed the highest inhibition effect on B. subtilis. and B. megaterium.. This extract also inhibited the growth of Gram-negative bacteria such as E. coli. and P. mirabilis.. When the inhibition zones obtained from R. fastigiata. were compared with that of standard antibiotic, it was determined that E. coli. and P. mirabilis. were more susceptible to the lichen extract. All the bacteria were found to be less susceptible to the acetone extracts obtained from other Ramalina. species than from Ramalina fastigiata.. B. subtilis. seems to be sensitive to the acetone extracts of all tested Ramalina. species.
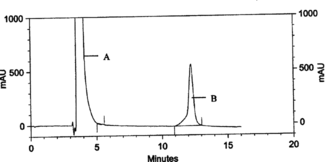
After that, we continued to determine the antimicrobially active substance usnic acid in an acetone extract of Ramalina. genus. Quantitative analysis of usnic acid in Ramalina capitata., R. polymorpha., R. fraxinea., R. pollinaria., and R. fastigiata. was achieved using HPLC. Identification of peaks in chromatograms of lichen extracts is accomplished by comparison of retention times with that of standard usnic acid. A sample of representative chromatograms is shown in . Usnic acid amounts and retention times in the acetone extracts of R. capitata., R. polymorpha., R. fraxinea., R. pollinaria., and R. fastigiata. are given in . The highest amount of usnic acid is found to be about 3.23% of the dry lichen weight in R. fastigiata..
Table 2.. Usnic acid content and retention times of lichen species.
Discussion
In recent years, a number of studies have focused on usnic acid. Usnic acid is a secondary metabolite that has an antimicrobial activity. Usnic acid has been shown to exhibit activity against clinical isolates of Enterococcus faecalis. and E. faecium. and clinical isolates of Staphylococcus aureus., including strains resistant to methicillin and mupirocin (Lauterwein et al., Citation1995). It also showed significant activity against pathogenic anaerobic Gram-negative bacilli (Bacteroides. spp.) and anaerobic Gram-positive bacteria (i.e., Clostridium. and Propionibacterium. spp.). Antifungal activity has been ascribed to usnic acid against the plant pathogens Penicillium frequentans. and Verticillium alboatrum. (Proksa et al., Citation1996). A diastereomeric mixture of dihydrousnic acids showed a broader spectrum of activity, inhibiting growth of Penicillium cyclopium., Penicillium frequentans., Talaromyces flavus., and Trichosporon cutaneum. while the 1-phenyl and 1-(N.-isonicotinoyl)-hydrazones were inactive. Due to the effects of Gram-positive organisms, mainly responsible for the development of body odor, usnic acid has been commercially used in deodorant sprays (Bergerhausen, Citation1976). Chinese researchers have shown usnic acid to exhibit inhibitory effects in vitro. against the pathogenic protozoan Trichomonas vaginalis. at slightly lower concentrations than metronidazole (Wu et al., Citation1995). Further, usnic acid is reported to be more effective than penicillin salves in the treatment of external wounds, burns, and is also used to combat tuberculosis (Nash, Citation1996). In this study, the antimicrobial activity of acetone extracts of lichens was tested against different Gram-positive cocci, bacilli, and Gram-negative bacilli. From our results, it could be concluded that Gram-positive bacilli are inhibited effectively in general.
High-performance liquid chromatographic has become more widely used as an effective analytical tool for the separation and identification of lichen substances (Feige et al., Citation1993). Several publications deal with the identification of lichen substances. Yoshimura et al. (Citation1994) described the use of a photodiode array detector for HPLC analysis of lichen substances (Yoshimura et al., Citation1994). According to Venkataramana et al. (Citation1992), a high-performance liquid chromatographic method for the determination of usnic acid in human plasma using diclofenac sodium as internal standard is described. Plasma proteins were precipitated with methanol. The method was applied to the determination of plasma levels of usnic acid after intravenous and oral administration to study its disposition in a healthy male rabbit. The HPLC method presented here is simple, selective, and sufficiently sensitive to determine plasma concentrations of usnic acid (Venkataramana et al., Citation1992).
Esimone and Adikwu (Citation1999) reported that the water, ethanol, chloroform, and n.-hexane extracts of the lichen Ramalina farinacea. showed antimicrobial activity against S. aureus., B. subtilis., E. coli., P. aeruginosa., and Salmonella typhi.. All the tested extracts showed antibacterial properties, bioactivity being in the order EtOH > n.-hexane > CHCl3 > water extract (Esimone & Adikwu, Citation1999). Also, Tay et al. (Citation2004) evaluated the acetone extract of the lichen Ramalina farinacea. in Turkey and showed antimicrobial activity against B. subtilis., Listeria monocytogenes., P. vulgaris., S. aureus., Streptococcus faecalis., Yersinia enterocolitica., Candida albicans., and C. glabrata.. They demonstrated that (+)-usnic acid, a constituent of Ramalina farinacea., is the major antimicrobial agent in this lichen (Tay et al., Citation2004). When the inhibition zones obtained from R. farinaceae. in this study were compared with Ramalina. species in our study, maximum antibacterial efficiency among all Ramalina. species in Turkey was exhibited by Ramalina farinaceae..
The in vitro. susceptibility of pathogenic Gram-positive bacteria, anaerobic bacteria, mycobacteria, and some fungi toward (+)- and (−)-usnic acids has been confirmed by Ingolfsdottir (Citation2002) and Lauterwerwein et al. (Citation1995). Lauterwerwein et al. (Citation1995) determined in vitro. activities of (+)-usnic acid, (−)-usnic acid, and vulpinic acid against aerobic and anaerobic microorganisms. They found that these lichen compounds did not inhibit Gram-negative rods or fungi at concentrations lower than 32 µg/mL, but were active against clinical isolates of Enterococcus faecalis., Enterococcus faecium., and Staphylococcus aureus. (Lauterwerwein et al., Citation1995).
According to Proksa et al. (Citation1996) usnic acid is widely distributed in species of Cladonia. (Cladoniaceae), Usnea. (Usneaceae), Lecanora. (Lecanoraceae), Ramalina. (Ramalinaceae), Evernia, Parmalia. (Parmeliaceae), and other lichen genera (Proksa et al., Citation1996). Alectoria. (Alectoriaceae) species are often rich sources of usnic acid, and yields of up to 6% have been reported (Proksa et al., Citation1996). In the current study, Ramalina fastigiata. yielded the highest usnic acid content with the value of 3.23%. This lichen species was collected from Antalya, located in the Mediterranean region. Also, the results obtained from this study are consistent with the results reported by Proska et al. (Citation1996).
As it can be seen from literature, the high antimicrobial activity of usnic acid has long been known, and our results show similar findings for the antimicrobial activity of usnic acid. The maximum antibacterial efficiency among five Ramalina. species was exhibited by Ramalina fastigiata., which has the highest usnic acid level. Although the literature contains many studies, this is the first report about usnic acid content of Ramalina. spp. in Turkey.
Acknowledgments
This study was partially supported by Ankara University, BITAUM. The authors are thankful to Prof. Dr. Ender Yurdakulol, for support in every aspect of the study.
References
- Behera BC, Adwadkar B, Makhija U (2003): Inhibitory activity of xanthine oxidase and superoxide-scavenging activity in some taxa of the lichen family Graphidaceae. Phytomedicine 10: 536–543.
- Bergerhausen H (1976): Deodorizing action of a complex of usnic acid. Cosm Toiletries 9: 25–26.
- Crittenden PD, Porter N (1991): Lichen-forming fungi: Potential sources of novel metabolites. Tibtech 9: 409–415.
- Esimone CO, Adikwu MU (1999): Antimicrobial activity and cytotoxicity of Ramalina farinacea.. Fitoterapia 70: 428–431.
- Feige GB, Lumbsch HT, Huneck S, Elix JA (1993): Identification of lichen substances by a standardized high-performance liquid chromatographic method. J Chromatogr 646: 417–427.
- Ingolfsdottir K (2002): Molecules of interest usnic acid. Phytochemistry 61: 729–736.
- Lautwerwein M, Oethinger M, Belsner K, Peters T, Marre R (1995): In vitro. activities of the lichen secondary metabolites vulpinic acid, (+)-usnic acid, and (−)-usnic acid against aerobic and anaerobic microorganisms. Antimicrob Agents Chemother 39: 2541–2543.
- Lawrey JD (1989): Lichen secondary compounds: Evidence for a correspondence between antiherbivore and antimicrobial function. Bryologist 92: 326–328.
- Miao V, Coffet-LeGal MF, Brown D, Sinnermann S, Donaldson G, Davies J (2001): Genetic approaches to harvesting lichen products. Trends Biotech 19: 349–355.
- Müller K (2001): Pharmaceutically relevant metabolites from lichens. Appl Microbiol Biotechnol 56: 9–16.
- Nash TH (1996): Lichen Biology. Cambridge, Cambridge University Press, pp. 1–289.
- Perry NB, Benn MH, Brennan NJ, Burgess EJ, Ellis G, Galloway DJ, Lorimer SD, Tangney RS (1999): Antimicrobial, antiviral and cytotoxic activity of New Zealand lichens. Lichenologist 31: 627–636.
- Proksa B, Sturdíková M, Prónayová N, Liptaj T (1996): (−) Usnic acid and its derivatives. Their inhibition of fungal growth and enzyme activity. Pharmazie 51: 195–196.
- Tay T, Türk AÖ, Yιlmaz M, Türk H, Kιvanç M (2004): Evaluation of the antimicrobial activity of the acetone extract of the lichen Ramalina farinacea. and its (+) usnic acid, norstictic acid, and protocetraric acid constituents. Z Naturforsch 59c: 384–388.
- Venkataramana D, Krishna DR (1992): High-performance liquid chromatographic determination of usnic acid in plasma. J Chromatogr 575: 167–170.
- Wu J, Zhang M, Ding D, Tan T, Yan B (1995): Effect of Cladonia alpestris. on Trichomonas vaginalis in vitro. Chinese.. J Parasit Parasit Dis 13: 126–129.
- Yamamoto Y, Kinoshita Y, Matsubara H (1998): Screening of biological activities and isolation of biological active compounds from lichens. Rec Res Dev Phytochem 2: 23–34.
- Yoshimura I, Kinoshita Y, Yamamoto Y, Huneck S, Yamada Y (1994): Analysis of secondary metabolites from lichen by high performance liquid chromatography with a photodiode array detector. Phytochem Anal 5: 195–205.