Abstract
A mixture of triterpenes named α.- and β.-amyrin (AMI), isolated from the Brazilian medicinal herb Protium hetaphyllum. (Aubl) March (Burseraceae), was evaluated for the ability to inhibit aggregation of human platelets induced by adenosine 5′-diphosphate (ADP, 1.5 and 3 µM), collagen, and arachidonic acid (AA) in vitro.. The results showed that AMI significantly inhibited platelet aggregation (40, 64, and 60%) in the assay carried out with ADP (3 µM) as agonist, at the doses of 100, 150, and 200 µM, respectively. In the presence of a lower ADP concentration (1.5 µM), a 3-time higher percentage of inhibition (32%) was observed with AMI 50 µM, as compared to that seen with 3.0 µM ADP. In the test using collagen (10 µg/mL) as agonist, AMI (50, 100, and 150 µM) inhibited aggregation by 26, 47, and 39%, respectively, while in the presence of the arachidonic acid (150 µM) at the doses of 50, 100, 150, and 200 µM, it inhibited platelet aggregation by 20, 21, 25, and 27%, respectively. The lowest IC50 value for the AMI inhibitory effect was observed with collagen (90.0 µM), followed by ADP (117.9 µM) and arachidonic acid (181.4 µM). With ADP as agonist, the anti-aggregant effect of the acetylsalicylic acid (ASA) was potentiated by AMI but not by dipyridamole. No potentiation was observed after the combination of ASA and AMI with collagen or arachidonic acid as agonists. Our results indicated that AMI possesses a platelet anti-aggregant activity in a concentration-dependent manner and probably acts on a biochemical pathway common to all the agonists tested.
Introduction
Protium heptaphyllum. Aubl. March (Burseraceae) is a medicinal plant, largely used in Brazil for its anti-inflammatory and anti-ulcer activities. Resins and leaves from Protium. species are commonly used in Brazil and elsewhere. The resin oil contains mainly monoterpenes and phenylpropanoids, whereas sequiterpenes predominate as the volatile constituents of the leaves (Siani et al., Citation1999). From the neutral fraction of the resin from Protium heptaphyllum. (Susunaga et al., Citation2001), mixtures of α.- and β.-amyrin, as well as of maniladiol and brein, were isolated as the main components. Terpenes are probably the most widespread chemical group of natural products, found mainly in plants as constituents of essential oils, whose structure is based on various isoprene units (methylbuta-1,3-diene or hemiterpene with 5 carbon atoms). This leads to a rational classification of terpenes, depending upon the number of such isoprene (or isopentane) units incorporated into the basic molecular skeleton. Many of these compounds occur in plants, as glycosides, which are molecules made up of carbohydrates linked to steroids or triterpenes. The triperpenes, β.- and α.-amyrin, can be isolated from several medicinal plants, including Protium heptaphyllum., and with lupeol constitute the three main groups of pentacyclic triterpenes.
Preliminary results (Aragão et al., Citation2002) showed that the mixture of α.- and β.-amyrin presents not only antinociceptive but also a potent anti-inflammatory activity in two different experimental models. An earlier study demonstrated that the palmitate of α.-amyrin exerts a strong anti-arthritic action (Kweifio-Okai et al., Citation1995), while others observed that α.-amyrin inhibited protein kinase from eukaryotic cells (Hasmeda et al., Citation1999). It has been recently shown (Otuki et al., Citation2005) that α.- and β.-amyrin isolated from the resin of Protium kleinii. Cuatrecas produces a consistent peripheral, spinal, and supraspinal antinociception in rodents, in inflammatory models of pain. According to these authors, the mechanisms of action may involve inhibition of protein kinase A and protein kinase C sensitive pathways.
Recent work also demonstrated antinociceptive as well as gastroprotective and anti-inflammatory properties in the resin from P. heptaphyllum.. These results indicate that the antinociceptive potential of α.- and β.-amyrin, main components of the resin, possibly involves the opioid and vanilloid receptor mechanisms (Oliveira et al., Citation2005; Lima-Junior et al., Citation2006). Furthermore, the gastro-protective mechanisms of the α.- and β.-amyrin mixture involves, at least in part, the activation of capsaicin-sensitive primary afferent neurons (Oliveira et al., Citation2004).
Several natural compounds, including terpenes, are known to produce inhibition of platelet aggregation, presenting a potential use as antithrombotic agents (Lin et al., Citation2001; Yang et al., Citation2002; Franscichetti et al., Citation1997; Li et al., Citation2002). Recently (Shen et al., Citation2000), spiramine Q, a diterpene isolated from the Chinese herbal plant Spiraea japonica. L. (Rosaceaea), was shown to selectively inhibit arachidonic acid-induced platelet aggregation, in vitro. or in vivo., and to decrease serotonin secretion from rabbit platelets. Their results demonstrated that spiramine Q has potent antiplatelet and antithrombotic activities. Chen et al. (Citation2001) showed that several compounds isolated from Corydalis tashiroi. Makino (Fumariaceae) possess antiplatelet aggregation activity. Recently, rubialbonol A and B and rubiarbonone A, isolated from Rubia yunnanensis. Diels (Rubiaceae), among other new triterpenoids, were demonstrated to present antiplatelet aggregation activities (Liou & Wu, Citation2002).
A recent work (Li et al., Citation2002) carried out with diterpene alkaloids from Spiraea japonica. L. (Rosaceae) showed that these compounds significantly inhibited platelet aggregation factor (PAF) induced platelet aggregation in a concentration-dependent manner, but had no effect on ADP or arachidonic acid-induced aggregation, exhibiting a selective inhibition. Their results suggest that these diterpene alkaloids are a class of novel antiplatelet aggregation agents.
The objectives of the present work, besides continuing our previous studies (Aragão et al., Citation2002) with P. heptaphyllum., are to investigate possible platelet inhibitory properties of the mixture of α.- and β.-amyrin isolated from this species, as well as to attempt the elucidation of its mechanism of action.
Materials and Methods
Isolation of α.- and β.-amyrin
The plant was collected in September 1998 in the city of Crato, in the state of Ceará, Brazil, and was identified by Prof. A. G. Fernandes of the Department of Biology of the Federal University of Ceará. The voucher specimen is deposited at the Prisco Bezerra Herbarium of the same university, under the number 28509. To obtain the resin, incisions were made on the plant stem. The resin (20 g) was fractionated by chromatography on a silica gel column, with hexane, chloroform, ethyl acetate, and methanol. The fraction obtained with chloroform (5.2 g) was chromatographed on a silica gel column and eluted with hexane and mixtures of hexane-ethyl acetate of increasing polarity. Fractions obtained from the hexane-ethyl acetate (1:1) mixture were chromatographed on a silica gel column, resulting in 450 mg of α.- and β.-amyrin (). Their structural identification was made by infrared spectrophotometry (KBr) v.max cm−1 (3300, 1480, and 1050) and nuclear magnetic resonance spectroscopy, NMR 1H (500 MHz, CDCl3) and NMR13C (125 MHz, CDCl3), with melting point 179–181°C, according to the literature (Mahato & Kundu, Citation1994). The relation of two to one α.- and β.-amyrin in the mixture was confirmed by NMR 1H.
Sample preparation
The mixture of α.- and β.-amyrin is a white amorphous powder, presenting a slight odor and low aqueous solubility, although it is soluble in organic solvents. In the present work, the mixture was suspended in 0.5% Tween 80, with pH = 6.0 and sonicated before use.
Reagents
Drugs and reagents were purchased from the chemical companies shown in parentheses. The following drugs and reagents were used: adenosine 5′-diphosphate (Sigma, USA), collagen type I (Biopool, Brazil), arachidonic acid (Sigma, USA), acetylsalicilic acid (Sigma, USA), sodium hydrogen carbonate (Reagen, Brazil), and Tween 80 (Reagen, Brazil). All other drugs were of analytical grade.
Human blood
The blood was collected in glass tubes, containing 3.8% sodium citrate (9:1, v/v), by venous puncture from healthy, non-smoking individuals who had not been taking any drug for at least two weeks. Assays were always performed around 3 h after blood collection. This study was previously approved by the Ethical Committee of the Faculty of Medicine of the Federal University of Ceará, Brazil.
Platelet aggregation assay in vitro.
The platelet anti-aggregant activity was determined by turbidimetry through an aggregometer model 450, from Chrono-log Corporation, USA, connected to a register. Initially, the platelet-rich plasma (PRP) was separated by centrifugation of a blood sample (twice, at 1000 rpm, for 6 min) at 25°C. The platelet-poor plasma (PPP) was then separated, after centrifugation of the remaining sample at 3000 rpm for 15 min at 25°C. Platelet aggregation assays were performed according to the method described by Born and Cross (Citation1963). Briefly, a 450 µl aliquot of the platelet suspension (PRP) was stirred at 1200 rpm and activated by the addition of different agonists with or without antagonists. The extent of aggregation was estimated quantitatively by measuring the maximum curve height. The inhibitory action of AMI was determined in the presence of ADP (3.0 and 1.5 µM), collagen (10 µg/mL), or arachidonic acid (150 µM) as agonists. We utilized a total of 22 samples of platelet-rich plasma. To each AMI concentration tested, the time of incubation ranged from 10 to 15 min at 37°C, followed by the agonist addition to the reaction medium.
Statistical analysis
Data are expressed as mean±SEM, and the statistical significance was determined using analysis of variance (ANOVA) followed by the Tukey's test. Values were considered significant at p < 0.05.
Results
In order to discard a possible interference of Tween 80 used for AMI dissolution, this vehicle alone was used as a control in the platelet aggregation curve. In addition, the relation between the anti-aggregant effect and the time of incubation was also determined, at several time points, showing that the anti-aggregant effect is unaltered after a 10 min incubation of AMI with PRP (data not shown). Our results demonstrated that AMI (100, 150, and 200 µM) produced inhibition of human platelet aggregation of the order of 40, 64, and 60%, respectively, in the presence of ADP (3 µM) as the agonist, while no significant inhibition was observed with the concentration of 50 µM. With a lower concentration of ADP (1.5 µM), a 3-time higher percentage of inhibition (28%) was observed with AMI 50 µM, as compared to that seen in the presence of 3 µM ADP (). With collagen as the agonist, AMI produced significant inhibition with concentrations as low as 25 µM. Thus, percentages of inhibition were 15, 26, 53, and 41%, with AMI concentrations of 25, 50, 100, and 150 µM, respectively (). Interestingly, as occurred in the presence of ADP, AMI at the highest concentration caused percentages of inhibition somewhat lower, suggesting a bell-shaped type of concentration-dependent response curve. Lower percentages of inhibition were observed with AMI in the presence of arachidonic acid, with no clear indication of a concentration-dependent response. The effect ranged from 15 to 27% inhibitions with the concentrations of 25, 50, 100, 150, and 200 µM (). presents IC50 values for AMI inhibitory effects on platelet aggregation induced by ADP, collagen, and arachidonic acid as agonists. The results showed that the lowest IC50 value was observed with collagen (90 µM), followed by ADP (117.9 µM) and arachidonic acid (181.4 µM), indicating that AMI is more effective in the presence of collagen as the agonist.
Table 1.. Inhibitory effects of alpha- and beta-amyrin (AMI) from Protium heptaphyllum. on human platelet aggregation induced by ADP.
Table 2.. Inhibitory effects of alpha- and beta-amyrin (AMI) from Protium heptaphyllum. on human platelet aggregation induced by collagen (10 µg/mL).
Table 3.. Inhibitory effects of alpha- and beta-amyrin (AMI) from Protium heptaphyllum. on human platelet aggregation induced by arachidonic acid (150 µM).
The potentiation of the ASA anti-aggregant effect by AMI, with two concentrations of ADP, is presented in . We showed that, in the presence of 3 µM ADP, the combination of ASA (25 µM) with AMI (25 µM) produced a 40% inhibition of platelet aggregation, while these compounds alone induced around 10% inhibitions. However, at a lower ADP concentration (1.5 µM), the association of ASA (12.5 µM) with AMI (25 µM) resulted in 59% inhibition of the platelet aggregation, while these compounds alone caused inhibitions of 13 and 20%, respectively, indicating a potentiation of the AMI effect by ASA. On the other hand, no alteration in the platelet aggregation by ADP was observed after the association of dipyridamole and AMI (data not shown). Furthermore, no significant potentiation of the AMI effect was demonstrated, after its combination with ASA, in the presence of collagen or arachidonic acid as agonists ().
Figure 2 Potentiation by AMI of the inhibitory effect of ASA on human platelet aggregation induced by ADP 3 and 1.5 µM.
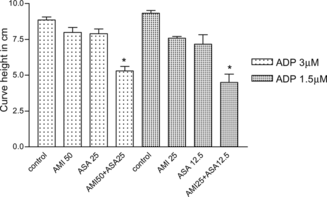
Table 4.. Potentiation by alpha- and beta-amyrin (AMI) from Protium heptaphyllum. of the inhibition of ASA-induced platelet aggregation in the presence of ADP, collagen, and arachidonic acid as agonists.
Table 5.. Values of IC50 for alpha- and beta-amyrin (AMI) from Protium heptaphyllum. on human platelet aggregation induced by ADP, arachidonic acid, and collagen as agonists.
Discussion
Several classes of terpenes, including triterpenes, isolated from natural sources, are known to produce inhibition of platelet aggregation. Ekimoto et al. (Citation1991) showed that several tetranotriterpenoids, isolated from Swietenia mahogany. L. Jacq. (Meliaceae), strongly inhibited PAF-induced aggregation, while glycyrrhizin, an anti-inflammatory compound from Glycyrrhiza glabra. Linne, has been identified as a new thrombin inhibitor (Franscichetti et al., Citation1997). These authors suggested that the anti-inflammatory effect of the compound may be due to its effective antithrombin action.
Some diterpenoids are also known to inhibit platelet aggregation, such as abietanes from Salvia miltiorrhiza. Bunge (Labiatae), which selectively inhibited rabbit platelet aggregation induced by arachidonic acid (Lin et al., Citation2001). Other diterpenoids, isolated from Annona squamosa. L. (Annonaceae), showed complete inhibitory effects on rabbit platelet aggregation (Yang et al., Citation2002). α.- and β.-Amyrin, isolated from several medicinal plants, were shown to present antinociceptive (Spessoto et al., Citation2003), irritant (Saeed & Sabir, Citation2003), cytotoxicity (Chaturvedula et al., Citation2002), and anti-ulcer properties (Navarrete et al., Citation2002). On the other hand, as far as we know, there are no reports in the literature on the anti-aggregant properties of α.- and β.-amyrin triterpenes.
In the present work, we demonstrate that α.- and β.-amyrin isolated from P. heptaphyllum. significantly inhibited, in a concentration-dependent manner, human platelet aggregation induced by ADP, collagen, or arachidonic acid. Platelet activation involves coordinated events that are tightly regulated. These events lead to a conformational change in the extracellular domain of the integrin platelet glycoprotein (GP)IIb-IIIa, facilitating the binding of soluble fibrinogen and enabling the formation of cross bridges between platelets, essential for platelet aggregation (Clutton et al., Citation2001).
Platelet aggregation can be induced through G protein-coupled receptors, responding to agonists such as ADP and thrombin, among others. ADP is an agonist that causes platelet shape change and aggregation, as well as generation of thromboxane A2, another agonist, through effects on P2Y1, P2Y12, and P2X1 receptors. ADP plays a key role in hemostasis, as it stimulates platelet aggregation and, when secreted from platelet-dense granules, potentiates the aggregation response induced by other agents. The sequence of events leading to ADP release is a complex one. After ADP addition, not only platelet aggregation but also the alterations in platelet shape contribute to the so called primary phase of aggregation, which is reversible and not release dependent. The secondary phase of platelet aggregation is irreversible and associated with the release of ADP, ATP, and 5-HT, which are responsible for the potentiation of the response.
A strong inhibition of platelet aggregation was observed in the presence of collagen, and a maximum effect was observed with the 150 µM concentration of AMI. Interestingly, in the presence of ADP or collagen, AMI produced a concentration-dependent effect up to a certain concentration range. However, at higher concentrations, a lower anti-aggregant effect was detected, characterizing a bell-shaped curve. Meei-Jen et al. (2002) showed that other triterpenes, such as rubiarbonone A and rubiarbonol A, promoted platelet aggregation at high concentrations. However, these compounds also exhibited a higher antiplatelet activity at lower concentrations, similar to the data shown in the present work.
Several types of collagen present in the sub-endothelial region, after injury, become exposed on the vascular surface and exert an important role in platelet activation, which is responsible for lesion repair. Monomeric collagen as well as fibrillar collagen can induce vascular injury, but only the adhesion of fibrillar collagen results in secretion. Collagen is known (Hubbard et al., Citation2003) to stimulate cell protein tyrosine phosphorylation and intracellular mobilization of calcium. Collagen-stimulated platelet activation leads to various events in signaling, generated by its interaction with the glycoprotein VI receptor. An earlier study (Pignatelli et al., Citation1998) suggested that collagen-induced platelet aggregation is associated with a burst of H2O2 that acts as a second messenger by stimulating the arachidonic acid metabolism and phospholipase C pathway. Gottstein et al. (Citation2003) showed that collagen-induced platelet aggregation, associated with a burst of hydrogen peroxide, contributes to the activation of platelet function through calcium mobilization and inositol pathway activation.
Arachidonic acid (AA) is a potent inducer of platelet aggregation in vitro., and this activity is due to its conversion to biologically active metabolites, as prostaglandins, endoperoxides and thromboxane A2 (TXA2), which are thought to act on the same receptor (Vezza et al., Citation2002). AA is metabolized via cyclooxygenase and thromboxane synthase, producing TXA2, a potent vasoconstrictor, and also inducing secretion and aggregation. TXA2 receptor stimulation activates phospholipase C and increases [Ca2+] via Gq protein, leading to aggregation by Ca2+ influx (Ohkubu et al., Citation1996). Saeed et al. (Citation2004) showed that sub-threshold concentrations of epinephrine potentiate platelet aggregation mediated by AA. According to them, the synergism may derive from the PLC/Ca2+, COX, and MAP kinase pathways and is negatively modulated by a nitric oxide donor. Our results demonstrate that the inhibition of platelet aggregation by AMI, in the presence of AA as agonist, ranged from 15 to 27% and was not as potent as that observed with ADP and collagen.
The antiplatelet effect of AMI was potentiated by acetylsalicylic acid (ASA) but not by dypiridamole. ASA is an antiplatelet agent that inhibits platelet cyclooxygenase 1 (COX1) and, as a consequence, prevents the formation of the pro-aggregatory substance TXA2. Dipyridamole has a direct antiplatelet activity in the platelet-rich plasma and whole blood when the cyclooxygenase pathway is blocked by ASA (Violi et al., Citation1991). Dipyridamole inhibits phosphodiesterase and adenosine deaminase activities and increases cAMP and adenosine, consequently inhibiting platelet aggregation (Fitzgerald, Citation1987). Considering that AMI potentiated the inhibitory activity of ASA with ADP, we suggest that the AMI mechanism of action might involve a greater activation of ADP receptors. Finally, although AMI inhibited human platelet aggregation in the presence of all three agonists, its effect was somewhat greater with ADP and mainly with collagen, as indicated by the lowest IC50 value of AMI when collagen was the agonist. However, AMI seems to act as a platelet antiaggregant, through pathways common to these three agonists. A similar mechanism in the synergism between various platelet agonists is thought to be due to the activation of Ca2+ signaling cascade. A rise in intracellular Ca2+ concentration, induced by the first agonist, primes platelets for an enhanced functional response to the second agonist (Saeed et al., Citation1997Citation2004). A similar effect may also explain, at least partly, the mechanism of action of α.- and β.-amyrin as platelet inhibitors.
Acknowledgments
The authors are grateful to the technical assistance of Ms. M. Vilani Rodrigues Bastos and to Prof. M.O.L. Viana for the orthographic revision of the manuscript. The work received financial support from the Brazilian National Research Council (CNPq).
References
- Aragão GF, Pinheiro MCC, Bandeira PN, Lemos TLG, Viana GSB (2002): Efeito antiinflamatório da fração isomérica de α.- e β.-amirina isolada de Protium heptaphyllum. (Aubl) March. XVII Simpósio de Plantas Medicinais do Brasil.
- Born GVR, Cross MJ (1963): The aggregation of blood platelets. J Physiol 168: 178–195.
- Chaturvedula VS, Schilling JK, Miller JS, Andriantsferana R, Rasamison VE, Kingston DG (2002): Two new triterpene esters from the twigs of Brachylaena ramiflora. from the Madagascar rainforest. J Nat Prod 65: 1222–1224.
- Chen JJ, Chang YL, Teng CM, Lin WY, Chen YC, Chen IS (2001): A new tetrahydroprotoberberine N.-oxide alkaloid and anti-platelet aggregation constituents of Corydalis tashiroi.. Planta Med 67: 423–427.
- Clutton P, Folts JD, Freedman JE (2001): Pharmacological control of platelet function. Pharmacol Res 44: 255–264.
- Ekimoto H, Irie Y, Araki Y, Han GQ, Kadota S, Kikuchi T (1991): Platelet aggregation inhibitors from the seeds of Swietenia mahagoni.: Inhibition of in vitro. and in vivo. platelet-activating factor-induced effects of tetranortriterpenoids related to swietenine and swietenolide. Planta Méd 57: 56–58.
- Fitzgerald D (1987). Drug therapy. Dipyridamole. N Engl J Med 316: 1247–1257.
- Franscichetti IM, Monteiro RQ, Guimarães JA, Francischetti B (1997): Identification of glycyrrhizin as a thrombin inhibitor. Biochem Biophys Res Commum 235: 259–263.
- Gottstein N, Ewins BA, Eccleston C, Hubbard GP, Kavanagh IC, Minihane AM, Weinberg PD, Rimbach G (2003): Effect of genistein and daidzein on platelet aggregation and monocyte and endothelial function. Brit J Nutrition 89: 607–616.
- Hasmeda M, Kweifio-Okai G, Macrides T, Polya GM (1999): Selective inhibition of eukaryote protein kinases by anti-inflammatory triterpenoids. Planta Med 65: 14–18.
- Hubbard GP, Stevens JM, Cicmil M, Sage T, Jordan PA, Williams CM, Lovegrove JA, Gibbins JM (2003): Quercetin inhibits collagen-stimulated platelet activation through inhibition of multiple components of the glycoprotein VI signaling pathway. J Thromb Haemost 1: 1079–1088.
- Kweifio-Okai G, Bird D, Field B, Ambrose R, Carroll AR, Smith P, Valdes R (1995): Antiinflammatory activity of a Ghanaian antiarthritic herbal preparation: III. J Ethnopharmacol 46: 7–15.
- Li L, Shen YM, Yang XS, Zuo GY, Shen ZQ, Chen ZH, Hao XJ (2002): Antiplatelet aggregation activity of diterpene alkaloids from Spiraea japonica.. Eur J Pharmacol 449: 23–28.
- Lima-Junior RC, Oliveira FA, Gurgel LA, Cavalcante IJ, Santos KA, Campos DA, Vale CA, Silva RM, Chaves MH, Rao VS, Santos FA (2006): Attenuation of visceral nociception by α.- and β.-amyrin, a triterpenoid mixture isolated from the resin of Protium heptaphyllum., in mice. Planta Med 72: 34–39.
- Lin HC, Ding HY, Chang WL (2001): Two new fatty diterpenoids from Salvia miltiorrhiza.. J Nat Prod 64: 648–650.
- Liou MJ, Wu TS (2002): Triterpenoids from Rubia yunnanensis.. J Nat Prod 65: 1283–1287.
- Mahato SB, Kundu AP (1994): 13C NMR Spectra of pentacyclic triterpenoids – A compilation and some salient features. Phytochemistry 31: 1517–1575.
- Meei-Jen L, Tiang-Shung W (2002): Triterpenoids from Rubia yunnanensis.. J Nat Prod 65: 1283–1287.
- Navarrete A, Trejo-Miranda JL, Reyes-Trejo L (2002): Principles of root bark of Hippocratea excelsa. (Hippocrataceae) with gastroprotective activity. J Ethnopharmacol 79: 2134–2139.
- Ohkubu S, Nakahat N, Ohizumi Y (1996): Thromboxane A2-mediated shape change: independet of Gq-phospholipase C–Ca2+ pathway in rabbit platelets. Br J Pharmacol 117: 1095–1104.
- Oliveira FA, Costa CL, Chaves MH, Almeida FR, Cavalcante IJ, Lima AF, Jr Lima RC, Silva RM, Campos AR, Santos FA, Rao VS (2005): Attenuation of capsaicin-induced acute and visceral nociceptive pain by α.- and β.-amyrin, a triterpene mixture isolated from Protium heptaphyllum. resin in mice. Life Sci 77: 2942–2952.
- Oliveira FA, Vieira-Junior GM, Chaves MH, Almeida FR, Santos KA, Martins FS, Silva RM, Santos FA, Rao VS (2004): Gastroprotective effect of the mixture of α.- and β.-amyrin from Protium heptaphyllum.: Role of capsaicin-sensitive primary afferent neurons. Planta Med 70: 780–782.
- Otuki MF, Ferreira J, Lima FV, Meyre-Silva C, Malheiros A, Muller LA, Cani GS, Santos AR, Yunes RA, Calixto JB (2005): Antinociceptive properties of mixture of α.-amyrin and β.-amyrin triterpenes: Evidence for participation of protein kinase C and protein kinase A pathways. J Pharmacol Exp Ther 313: 310–318.
- Pignatelli P, Pulcinelli FM, Lenti L, Gazzaniga PP, Violi F (1998): Hydrogen peroxide is involved in collagen-induced platelet activation. Blood 15: 484–490.
- Saeed SA, Gilani AH, Majoo RU, Shan BH (1997): Anti-thrombotic and anti-inflammatory activities of protopine. Pharmacol Res 36: 1–7.
- Saeed SA, Rasheed H, Fecto FAW, Achakzai MI, Ali R, Connor JD, Gilani A (2004): Signaling mechanisms mediated by G-protein coupled receptors in human platelets. Acta Pharmacol Sin 25: 887–892.
- Saeed MA, Sabir AW (2003): Irritant potencial of some constituents from the seeds of Caesalpinia bonducella. (L.) Fleming. J Asian Nat Prod Res 5: 35–41.
- Shen Z, Chen Z, Li L, Lei W, Hao X (2000): Antiplatelet and antithrombotic effects of the diterpene spiramine Q from Spirea japonica. var. incisa.. Planta Med 66: 287–289.
- Siani AC, Ramos MFS, Menezes-de-Lima JO, Santos RR, Ferreira EF, Soares ROA, Rosas EC, Susunaga GS, Guimarães AC, Zoghbi MGB, Henriques MGMO (1999): Evaluation of anti-inflammatory-related activity of essential oils from the leaves and resin of species of Protium.. J Ethnopharmacol 66: 57–69.
- Spessoto MA, Ferreira DS, Crotti AE, Silva ML, Cunha WR (2003): Evaluation of the analgesic activity of extracts of Miconia rubuginosa. (Melastomaceae). Phytomedicine 10: 606–609.
- Susunaga GS, Siani AC, Pizzolatti MG, Yunes RA, Delle Monache R (2001): Triterpenes from the resin of Protium heptaphyllum.. Fitoterapia 72: 709–711.
- Vezza R, Mezzasoma AM, Venditti G, Gresele P (2002): Prostaglandin endoperoxides and thromboxane A2 activate the same receptor isoforms in human platelets. Thromb Haemost 87: 114–121.
- Violi F, Pratico D, Iuliano L, Balsano F (1991): Dipyridamole potentiates the inhibition of platelet aggregation by aspirin (in human platelet rich plasma and whole blood). Lipid Mediat 4: 61–67.
- Yang YL, Chang FR, Wu CC, Wang WY, Wu YC (2002): New ent.-kaurane diterpenoids with anti-platelet aggregation activity from Annona aquamosa.. J Nat Prod 65: 1462–1467.