Abstract
The effects of the ethanol extract of Tilia cordata. Miller (Tiliaceae) were studied in vitro. using intestinal smooth muscle cells of guinea pigs dispersed by collagenase. The extract induced a dose-dependent contraction of the dispersed smooth muscle cells. The obtained data indicated a direct effect of the T. cordata. extract on the intestinal smooth muscle cells. In addition, the contractions induced by the extract were inhibited by atropine. These observations indicate involvement of some active constituents, with cholinergic properties, found in the alcohol extract to induce contraction of the intestinal smooth muscle cells via activation of the muscarinic receptors.
Introduction
Tilia cordata. Miller (Tiliaceae), also known as linden, small-leaved linden, and lime tree, is a tall decidious tree reaching up to 25 m. Linden, native throughout Europe, is cultivated in Europe and North America. The commercial products are obtained mainly from T. cordata. and Tilia platyphyllos. Scopoli (Tiliaceae). The latter is known as large-leaved linden. The yellowish white flowers of T. cordata. are arranged in clusters of 3–15 on a stalked erect cyme, half joined to a membranous bract occurring in the leaf axils. The flowers are characterized by numerous stamens; fruit is an ovoid hairy achene. The flowers are used as a medicinal plant for the treatment of different ailments externally and internally (British Herbal Pharmacopoeia., Citation1990; Tyler, Citation1993; Blumenthal et al., Citation2000). The use of the flowers of T. cordata. for therapeutic purposes dates back to the Middle Ages, and many pharmacologic as activities such as antitussive, diaphoretic, diuretic, sedative, antispasmodic, hypotensive, as well as emollient and mild astringent activities have been reported (Tyler, Citation1993; Jaenicke et al., Citation2003).
Lime flowers are rich in carbohydrates, amino acids, and different secondary metabolites of shikimic acid, acetate-malonate and mevalonic acid pathways. The presence of phenolic acids (caffeic, p.-coumaric, and chlorogenic acids), amino acids, mucilage polysaccharides, flavonoids (kaempferol, quercetin, myricetin and their glycosides such as rutin, hyperoside, quercitrin, and isoquercitrin), volatile oils (anethole, citral, citronellol, eugenol, limonene, menthone, nerol, α-pinene, terpineol, fenchone, α- and β-thujone, and farnesol), and tannins have been reported (British Herbal Pharmacopoiea., Citation1990; Buchbauer et al., Citation1992; Tyler, Citation1993; Newall et al., Citation1996; Vidal & Richard, 1996; Behrens et al., Citation2003; Jaenicke et al., Citation2003).
Many studies have been conducted to demonstrate effects of these active substances on smooth muscle cells. Most of these studies have demonstrated inhibitory contractile responses of vascular smooth muscle cells. The antihypertensive action of quercetin and relaxation of vascular muscle cells have been demonstrated as effects of the flavonoids (Duarte et al., Citation2001; Perez-Vizcaino et al., Citation2002; Ajay et al., Citation2003). Studies on contractile mechanisms of smooth muscle cells have indicated inhibition of myosine kinase activity, as well as Ca2+ influx and Ca2+ release from internal stores (Rogers & Williams, Citation1989; Ajay et al., Citation2003). Other studies have shown endothelium-dependent relaxation of vascular smooth muscle cells induced by quercetin and tannins (Andriambelosson et al., Citation1998; Flesch et al., Citation1998). Inhibitory effects on longitudinal muscles of ileum have also been reported for several flavonoids (Midleton et al., Citation2000). These responses may indicate a potential inhibitory effect of the extract of T. cordata. on gastrointestinal muscle.
Several experiments, carried out with the extracts prepared from different Tilia. species, demonstrated the activity of this genus on the intestine. Isolation of benzodiazepine receptor ligand from Tilia tomentosa. Moench (Tiliaceae) (Viola et al., Citation1994), detection of GABA (Cavadas et al., Citation1994), and sedation and anxiolytic effects induced by aqueous extract from T. europaea. L. (Figueiredo et al., Citation1994) showed effects of linden extracts on neural cells. In vitro. studies on intestinal preparation, using active material extracted from linden flowers (volatile oil, farnesol), have shown antispasmodic and sedative effects on rat duodenum (Lanza & Steinmetz, Citation1986). In another study, contractions caused by the aqueous extract of T. europaea. on guinea pig ileum were inhibited by atropine (Cotrim et al., Citation1994). Smooth muscle cell responses to inhibitory and excitatory neurotransmitters released from enteric neurons by neural stimulation may mask the direct effects induced on smooth muscle cells by the plant extract. Therefore, the current study was designed to investigate the direct effects that can be induced on dispersed smooth muscle cells from the small intestine by the ethanol extract of T. cordata..
Materials and Methods
Plant material
Commercially available dried flowers of T. cordata. were purchased in a local market in Ankara, Turkey, in summer 2004. The plant material was authenticated by Prof. Dr. B.E. Abu-Irmaileh, Faculty of Agriculture, University of Jordan, Amman. A voucher specimen was deposited at the Department of Pharmaceutical Sciences, Faculty of Pharmacy, University of Jordan (herbarium no.: 1 TILI-FMJ). Atropine (vials 0.6 mg/mL) used in the current study was produced by Arab Pharmaceuticals Manufacturing Co. (Jordan), collagenase by Worthington (USA), and acrolein by Sigma/Aldrich (Germany).
Extract preparation
Finely crushed and coarsely powdered flowers (100 g) were extracted by refluxing for 1 h using 1 L of 96% EtOH. The extract was kept overnight, filtered, and used for in vitro. experiments and thin layer chromatograpy (TLC) screening.
Screening of the extract by TLC
Different flavonoids and volatile oils were identified by comparison of their R-values and color reactions with authentic samples using specific solvent systems (SS) and spraying reagents (SR). Precoated TLC plates (Silica gel 60 with fluorescence UV254 thickness 0.2 mm; Albet, Barcelona, Spain) and analytical grade solvents were used. The presence of hyperoside, rutin (SS: ethyl acetate:formic acid:glacial acetic acid:water [100:11:11:27]; SR:FeCl3 and Naturstoff reagent), quercetin (SS:toluene:ether:glacial acetic acid [50:50:10]; SR:ferrichloride and Naturstoff reagent), α.-pinene, β.-pinene, and limonene (SS:toluene:ethyl acetate [93:7]; SR:10% vanillin–sulfuric acid) was confirmed. Additionally, the presence of an unidentified coumarin was recognized (SS:diethylether:toluene:formic acid [1:1:0.1]; SR:ethanolic potassium hydroxide). Solvent systems and spraying reagents were prepared according to Wagner and Bladt (Citation1996).
Dispersion of smooth muscle cells
Nine guinea pigs of both sexes, weighing 350–450 g, were obtained from the Experimental Animal Laboratory, University of Jordan, and kept for 1 week under observation. The animals were healthy and were fed standard laboratory diet. During the study, animals were processed according to the suggested ethical guidelines for the care of laboratory animals to minimize pain and discomfort. On the day of the experiment, fully ether anesthetized guinea pigs were sacrificed by opening of the chest cavity. The whole small intestine was removed and placed in freshly prepared Krebs solution previously aerated with 95% O2 and 5% CO2 and the pH adjusted to 7.4. Small intestine was cut into small pieces of 2–2.5 cm. After the removal of longitudinal layer and mucosa by mechanical scraping, the circular layer was taken and prepared for enzymatic digestion by chopping. Smooth muscle cells were dispersed by enzymatic digestion as described by Murthy et al. (Citation1991). Briefly, chopped preparations were placed into 30 mL Krebs solution containing 320 U collagenase/mL. The digestion process continued for about 90 min at 35°C with very low bubbling by 95% O2 and 5% CO2. Anliquot, containing dispersed smooth muscle cells after the process of digestion, was filtered through nitex mesh of 500 µm. The filtrate was centrifuged for 10 min at 3000 rpm. After this process, digestive solution was removed, and cells were resuspended in collagenase-free Krebs solution for washing and centrifuged twice. After the second wash, cells were suspended into Krebs solution and were ready for use in the current study.
Experimental protocol
Equal volumes of solution containing dispersed cells were placed in test tubes and divided in four groups: group 1 consisted of tubes containing only dispersed cells (control); group 2 consisted of tubes containing dispersed cells and atropine (control A); group 3 consisted of dispersed cells and T. cordata. extracts in different concentrations (1 mg, 0.5 mg, 50 µg, and 5 µg); and group 4 consisted of dispersed cells, atropine, and T. cordata. extracts in concentrations as in group 3. At 30 s, after addition of plant extract in groups 3 and 4, muscle activity was stopped with 1% acrolein in all groups. For micrometric scanning, dispersed cells were spread over microscopic slides, covered with cover slide, and kept in humid atmosphere at 4°C. Experiments were repeated for all groups, as reported in the Results, not less than five-times.
Measurement of dispersed smooth muscle cells
On the second day, the length of smooth muscle cells was measured by micrometric scanning technique as described by Murthy et al. (Citation1991). The mean length of the first 50 encountered cells was taken from each slide and considered as one experimental result.
Analysis of results
All data are presented as means of smooth muscle length±SEM. The differences between means of the control and extract treated cells were compared by using Student's t.-test. Significance was considered with p value of < 0.05.
Results
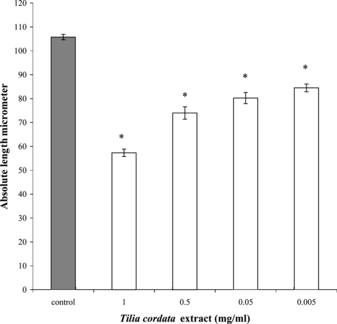
The ethanol extract from T. cordata. produced contraction over dispersed smooth muscle cells from the circular layer of guinea pig small intestine. In the control tube, the mean length of the smooth muscle cells from the small intestine was 105.8±1.3 µm (n = 9). At the highest concentration of extract (1 mg/mL), the mean length of smooth muscle cells was 57.3±1.6 µm (n = 6). At the concentration of 0.5 mg/mL, the mean length was 74.0±2.6 µm (n = 8). At 50 µg/mL, the length was 80.3±2.3 µm (n = 8), and at 5µg/mL, it was 84.5±1.6 µm (n = 7) (). In percentage, the decrease in muscle length was 54.0%, 69.9%, 75.9%, and 79.9% of that of control at extract concentrations of 1 mg, 0.5 mg, 50 µg, and 5 µg/mL; respectively.
Figure 1 Contractile effect of Tilia cordata. extract on smooth muscle cells. Results are means±SE of 6–9 experiments. Significance of difference from corresponding control value: *p < 0.05.
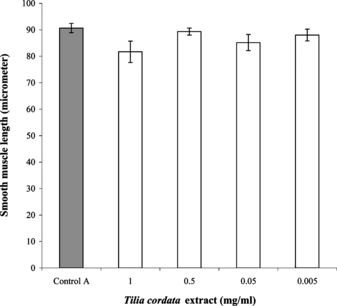
Cells that had been previously treated with atropine did not show much change in the length to extract treatment. The mean length of cells in the atropine control was 90.7±1.8 µm (n = 9). The mean length of cells treated with the highest concentration of T. cordata. extract (1 mg/mL) was 81.7±4.0 µm (n = 6). With extract concentration of 0.5 mg/mL, the mean length was 89.3±1.3 µm (n = 6), with 50 µg/mL, it was 85.2±3.1 µm (n = 6), and with 5 µg/mL, it was 88.0±2.2 µm (n = 5) (). In percentage, the mean length of smooth muscle cells was 90.0%, 98.5%, 93.9%, and 97.0% of that of atropine control at extract concentrations of 1 mg, 0.5 mg, 50 µg, and 5 µg/mL, respectively.
Discussion
The activity of smooth muscle cells of the gastrointestinal tract is influenced by neurotransmitters released from enteric neurons. These neurons are releasing excitatory and inhibitory neurotransmitters. In addition, non/adrenergic/non/cholinergic (NANC) enteric neurons can synthesize and release nitric oxide (NO), which can also inhibit contractile responses of smooth muscle cells (Torsoli et al., Citation1993). By dispersion of smooth muscle cells, the effects of T. cordata. extracts on smooth muscle cells that can be mediated by enteric neurons have been eliminated.
In the current study, different concentrations of the ethanol extract of T. cordata. caused significant (p < 0.05) contractions of the non-atropine treated dispersed smooth muscle cells of the small intestine of guinea pigs. The contractions induced were concentration dependent (). The obtained results clearly indicated that the substances found in the ethanol extract of T. cordata. induced the contraction of the intestinal smooth muscle cells. In an earlier study, Cotrim et al. (Citation1994) reported that the aqueous extract prepared from T. europaea. has also shown contractile responses of ileum segment from guinea pig. Similar results obtained in both studies indicate that substances responsible for the contraction of smooth muscles are rather polar substances, such as the flavonoid glycosides found in both species. However, the reported inhibition caused by quercetin on stimulated release of acetylcholine from enteric neurons indicates effects of T. cordata. on enteric neurons (Midleton et al., Citation2000). In addition, detection of GABA in aqueous extract of T. europaea. (Cavadas et al., Citation1994), isolation of benzodiazepine receptor ligands from T. tomentosa. (Viola et al., Citation1994), and anxiolytic and sedative effects exhibited by the aqueous extracts of T. europaea. (Figueiredo et al., Citation1994) also indicate strong effects of extracts of some representatives of the genus Tilia. on neural cells. In our study, by dispersion of cells, we have eliminated mediated responses of smooth muscle cells via enteric nervous system, and the contractile responses of intestinal muscle cells has almost been induced by direct effects of active materials found in ethanol extract.
Muscle cells that have been pretreated with atropine (2 µM) did not exhibit significant contractile response to the ethanol extract of T. cordata. (). The absence of contraction by these cells indicates that the active materials that caused contraction of smooth muscle cells have exerted their effect through muscarinic receptors. This may indicate cholinergic activity of active substances found in the ethanol extract of T. cordata.. It is not known whether the cholinergic activity of linden extract can also induce effects through nicotinic receptor. At enteric neurons, nicotinic receptors are involved in activation of NO synthesis (Hebeiss & Kilbinger, Citation1999). Activation of nicotinic receptor by T. cordata. extract, if present, can be considered at least in part in inhibitory mechanisms of smooth muscle cell activity mediated by enteric neurons.
Conclusions
The ethanol extract of T. cordata. induced contraction by direct effects on intestinal smooth muscle cells via activation of muscarinic receptors. Similar studies with the purified active constituents of T. cordata. will be the continuation of the current work to detect the substance(s) responsible for the observed biological activity.
Acknowledgments
This work was supported by a grant (689/2001) of the Deanship for Scientific Research, University of Jordan. The authors are grateful to Mr. Jalal Zeidan and Mr. Ismael Abaza for technical help.
References
- Ajay M, Anwar-ul Hassan G, Mustafa MR (2003): Effects of flavonoids on vascular smooth muscle of the isolated rat thoracic aorta. Life Sci 74: 603–612.
- Andriambelosson E, Magnier C, Haan-Archipoff G, Lobstein A, Anton R, Beretz A, Stoclet JC, Andriantsitohaina R (1998): Natural dietary polyphenolic compounds cause endothelium dependent vasorelaxation in rat thoracic aorta. J Nutr 128: 2324–2333.
- Behrens A, Maie N, Knicker H, Koegel-Knaber I (2003): MALDI-TOF mass spectrometry and PSD fragmentation as means for the analysis of condensed tannin in plant leaves and needles. Phytochemistry 62: 1159–1170.
- Blumenthal M, Goldberg A, Foster S (2000): Herbal Medicine–Expanded Commission E Monographs. American Botanical Council, Integrative Medicine Communications, Boston, MA, pp. 240–243.
- British Herbal Pharmacopoiea. (1990): Bourremouth, UK, British Herbal Medicine Association, Biddles Ltd., Vol. I, p. 62.
- Buchbauer G, Jirovetz L, Remberg B, Remberg G, Nikiforov A (1992): Headspace analysis of the dried herb of passion flower (Herba passiflorae.) and dried flowers of lime tree (Flores tiliae.). Flavour Fragr J 7: 329–332.
- Cavadas C, Fontes Ribeiro CA, Arujo I, Santos MS, Proenca da Cunha A, Macedo TRA, Caramona MM, Cotrim MD (1994): Influence of Tilia europaea. extracts on GABA receptor. Br J Pharmacol 114(Suppl): abstract 285P.
- Cotrim MD, Figueiredo IV, Cavadas C, Fontes Ribeiro CA, Isabel PJ, Proenca da Cunha A, Caramona MM, Macedo TRA (1994): Effects of Tilia europaea. on guinea pig ileum and aorta. Br J Pharmacol 114 (Suppl): abstract 287P.
- Duarte J, Perez-Palencia R, Vargas F, Ocete MA, Perez-Vizcaino F, Zarzuelo A, Tamargo J (2001): Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br J Pharmacol 133: 117–124.
- Figueiredo IV, Cotrim MD, Cavadas C, Fontes Ribeiro CA, Isabel PJ, Proenca da Cunha A, Caramona MM, Macedo TRA (1994): The potential anxiolytic activity of Tilia europaea. aqueous extract in mice. Br J Pharmacol 114(Suppl): abstract 286P.
- Flesch M, Schwarz A, Bohm M (1998): Effects of red and white wine on endothelium-dependent vasorelaxation of rat aorta and human coronary arteries. Am Physiol Heart Circul Physiol 275: H1183–1190.
- Hebeiss K, Kilbinger H (1999): Cholinergic and GABAergic regulation of nitric oxide synthesis in the guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 276: G862–G866.
- Jaenicke C, Gruenwald J, Brendler T (2003): Handbuch Phytotherapie. Stuttgart, Wissenschaftliche Verlagsgesellschaft mbH, pp. 329–330.
- Lanza JP, Steinmetz M (1986): Action comparees des extrait aqueux de graines de Tilia platyphylla. et de Tilia vulgaris. sur l'intestine isole de rat. Fitoterapia 57: 185.
- Midleton E Jr, Kandaswami C, Theoharides CT (2000): The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease and cancer. Pharmacol Rev 52: 673–751.
- Murthy KS, Grider JR, Makhlouf GM (1991): InsP3-dependent Ca2+ mobilization in circular but not longitudinal muscle cells of intestine. Am J Physiol 261: G937–G944.
- Newall CA, Anderson LA, Phillipson JD (1996): Herbal Medicines: A Guide for Health-care Professionals. London, The Pharmaceutical Press, pp. 181–182.
- Perez-Vizcaino F, Ibara M, Angel L, Cogolludo AL, Duarte J, Zaragoza-Arnaez F, Moreno L, Lopez-Lopez G, Tamargo J (2002): Endothelium-independent vasodilator effects of the flavonoid quercetin and its methylated metabolites in rat conductance and resistance arteries. J Pharmacol Exp Ther 302: 66–72.
- Rogers JC, Williams DL Jr (1989): Kaempferol inhibits light chain kinase. Biochem Biophys Res Comm 164: 419–425.
- Torsoli A, Annese V, Corzziari E, Cucchiara S, Renzi D, Severi C, Stanghellini V, Sternini C, Surrenti C (1993): Neuroendocrine regulation of gastrointestinal motility. Normal and abnormal physiology. Ital J Gastroenterol 25: 123–134.
- Tyler VE (1993): The Honest Herbal. London, Pharmaceutical Products Press, pp. 203–204.
- Vidal JP, Richard H (1986): Characterization of volatile compounds in linden blossoms Tilia cordata. Mill. J Flav Fragr 1: 57–62.
- Viola H, Wolfman C, Levi de Stein M, Wasowski C, Pena C, Medina JH, Paladini AC (1994): Isolation of pharmacologically active benzodiazepine receptor ligands from Tilia tomentosa. (Tiliaceae). J Ethnopharmacol 44: 47–53.
- Wagner H, Bladt S (1996): Plant Drug Analysis, A Thin Layer Chromatography Atlas. Berlin, Springer-Verlag, pp. 6–8, 145–147, 163–165.