Abstract
Teucrium polium. (Lamaceae), part of the natural flora of Iran and the Middle East, has long been used for the treatment of diabetes, gastric inflammation, and convulsion in traditional medicine (Reishinger, Citation). Although some benefits of T. polium. have been claimed by researchers, very few studies have investigated the cytotoxic effects of this herb. In the current study, we investigated the cytotoxic effects of an ethanol extract of T. polium. on four cell lines, A549, BT20, MCF7, and PC12, by measurement of mitochondrial respiration as well as colony-forming assay. We also compared T. polium. cytotoxicity to paclitaxel, a known herbal anticancer agent. Our results show that the ethanol extract of T. polium. suppressed the growth of all tested cell lines effectively. The IC50 values for each cell line were calculated as follows: A549, 90 µg/mL; BT20, 106 µg/mL; MCF-7, 140 µg/mL; and PC12, 120 µg/mL. T. polium. extract also inhibited formation of colonies in agarose efficiently. Further investigations are now needed to establish the exact mechanism of action and identify the active ingredient(s) of T. polium. extract in order to show its therapeutic efficacy.
Introduction
Cancer is a major, worldwide, life-threatening disease in both males and females. Many studies have been done and lots of investigations are in progress to find new anticancer agents. Chemotherapy side effects arising from chemotherapy-resistant kinds of neoplasm as well as decreased efficacy of chemotherapy agents express the importance of studies in finding new anticancer agents (Stewart & Coates, Citation2005). The role of herbal medicine as a source of new chemotherapy agents is always under consideration. Herbal medicine studies have led to discovery of chemotherapy agents such as paclitaxel (Taxol) and camptothecin.
Teucrium polium. (Lamaceae) grows in Iran and the Middle East as part of the natural flora (Reichinger., Citation1982; Abdollahi et al., Citation2003;). As a traditional remedy, T. polium. is used for treatment of convulsion, headache, and digestive diseases in Iran (Baluchnejadmojarad et al., Citation2005). T. polium. extract contains selenium (Jurisic et al., Citation2003) and has shown antioxidant activity (Ljubuncic et al., Citation2006) like α-tocopherol. Some studies state the anti-inflammatory effects of T. polium. extract (Tariq et al., Citation1989; Ljubuncic et al., Citation2005). Antibacterial activity against some Gram-positive and Gram-negative bacteria (Autore et al., Citation1984) as well as antifungal activity against Yarrovia lipolitica. and Saccharomyces cerevisiae. (Aggelis et al., Citation1998) has also been reported. An early study has shown that diterpenoids present in T. polium. extract have in vivo. antitumor activity in the P388 mouse model of leukemia (Nagao et al., Citation1982).
Despite these studies, no investigation has yet addressed the effects of T. polium. extract on in vitro. proliferation and survival of malignant cell lines. The current investigation aims to study the effects of an alcohol extract of T. polium. on proliferation and colony-forming efficacy of A549 (human lung adenocarcinoma), BT20 (human breast ductal carcinoma), MCF-7 (human breast adenocarcinoma), and PC12 (mouse pheochromocytoma) cell lines.
Materials and Methods
Plant material
The leaves of T. polium. were collected from Khabr (Kerman province, Iran). A voucher specimen was identified at the Department of Pharmacognosy, School of Pharmacy, Kerman University of Medical Sciences (Kerman, Iran) and deposited at the herbarium of Kerman School of Pharmacy under number 1011.
Extraction and preparation of plant sample
Powdered plant specimen (50 g) was extracted at room temperature with ethanol 96° by maceration method for 48 h, two times, separately. The ethanol extract was evaporated to near dryness by rotary evaporation at 40°C and was stored at 4°C protected from light until it was used in different cytotoxic assays. Immediately before assays were performed, the extract was dissolved in a small amount of dimethyl sulfoxide (DMSO) (Sigma-Aldrich, USA) up to 0.5% before diluting with medium.
Cell culture
A549, MCF-7, BT20, and PC12 cell lines were obtained from Iranian National Cell Bank, Pasteur Institute, Iran. A549, MCF-7, and BT20 cells were grown in DMEM/F12 (Sigma) and PC12 cells were grown in RPMI 1640 (Sigma) medium in a humidified incubator and an atmosphere of 5% CO2 in air at 37°C. All media were supplemented with 10% fetal bovine serum (FBS) (Biochrome, Australia), 100 U/mL penicillin, and 50 µg/mL streptomycin.
Cytotoxicity assays
Cells in exponential growth phase were trypsinized and resuspended in culture medium at density of 105 cells/mL for expansion. For cytotoxic assays, cells were plated at 104 cells/well in 96-well culture plates and left in the incubator overnight for adhesion. The extract was dissolved in DMSO and the concentration was adjusted to 50, 100, 150, and 200 µg/mL. The solution was added to each well. Cells were incubated for 48 h and then cellular proliferation was measured using WST-1 (Roche, Germany) cell proliferation kit according to the manufacturer's instructions; 10 µL of WST-1 was added to each well, and optical absorbance was measured both immediately (to exclude absorbance created by the color of solution) and after 1 h at 450 nm and reference wavelength of 630 nm with BIOTEK ELISA Reader (USA). We used paclitaxel (Sigma) as a positive control, and all tests were performed independently five times.
Colony-forming growth assay
Cells in exponential growth phase were trypsinized and resuspended in culture medium with 20% FBS and 0.25% type VII agarose (Sigma) to 500 cells/mL. One milliliter of this solution was added to each 3-cm Petri dish coated previously with culture medium supplemented with 20% FBS, 0.5% type VII agarose, and normal penicillin and streptomycin concentration. Twenty-four hours after cultivation, the extract was added to each dish, and dishes were returned to humidified CO2 incubator. One week later, 1 mL of the same medium was added to each dish.
After 14 days, colonies were counted, and the diameter of colonies was measured carefully using a calibrated eyepiece reticule on an inverted microscope (Nikon TS100, Japan). These tests were replicated three independent times.
Statistical analysis
Data are reported as mean ± SE. The data from WST-1 assays were analyzed statistically by ANOVA followed by post hoc test. Data from colony-forming assays were analyzed by Student's t.-test.
Results
WST-1 assay
The cytotoxicity of T. polium. ethanol extract was evaluated by using the WST-1 method. Established cell lines used in the current study were A549, BT20, MCF-7, and PC12. T. polium. extract was used at concentrations of 50, 100, 150, and 200 µg/mL. The experiments were replicated five times. T. polium. extract showed cytotoxicity against all tested cell lines; inhibitory concentration (IC50) values were calculated by Curvexpert 1.3 software as follows: 90 µg/mL for A549, 120 µg/mL for PC12, 106 µg/mL for BT20, and 140 µg/mL for MCF-7. T. polium. ethanol extract cytotoxicity was also compared with taxol cytotoxicity. The results for different doses of T. polium. on cell lines are summarized in .
Table 1. Effect of different doses of T. polium. on proliferation of some established cell lines after WST-1 assay
Colony-forming assay
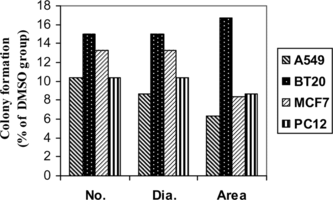
The effect of T. polium. ethanol extract on tested cell lines was studied using colony-forming assay. Our results showed that T. polium. extract highly reduced colony-forming efficacy in all the cell lines compared with both free and vehicle (DMSO) group. We also calculated the surface occupied by colonies for each treatment. Great differences were observed between treatment and control groups (). Colony formation efficacy, expressed as percentage of the number of colonies, the total diameter of colonies, and the total area occupied by colonies in experiment groups divided by the same figures in vehicle group, is shown in .
Table 2. Characteristics of colony-forming assays of different cell lines after treatment with 100 µg/mL T. polium..Footnotea.
Discussion
T. polium., as a natural herbal medicine has long been used in the Middle East for treatment of various diseases including headache, diabetes, and gastritis (Abdollahi et al., Citation2003). However, to our knowledge, this is the first report that explains the effects of T. polium. on survival and proliferation of some established cell lines.
Both conversion of tetrazolium bromide (WST-1 assay) to foramazan derivative by mitochondrial succinate dehydrogenase in viable cells as a rapid test and colony-forming assay as a slow-acting test resulted in significant decrease of cell activity after administration of T. polium. extract. However, long-term effects (colony-forming assay) of T. polium. were much more pronounced than the rapid test (WST-1). Very few colonies grew when 100 µg/mL crude ethanol extract of T. polium. was added to dishes containing each cell line. In addition, by close observation of treated colonies under inverted microscope, we found that the morphology of exposed colonies was abnormal. The exposed cells were vacuolated and dark granules were abundant in the cytoplasm. In addition, most of the cell membranes were disintegrated (data not shown).
Among the cell lines we used in the current study, A549 cells were more sensitive while PC12 cells were less sensitive to T. polium. extract. Such variation is also seen from the results of taxol; when 50 µg/mL taxol was used as positive control, the most sensitive cell line was BT20, while for PC12 cells it was even an enhancer. However, 150 µg/mL crude extract of T. polium. was much more effective than 50 µg/mL taxol (). Different nature of cell lines and their sensitivity to environmental factors may explain these differences.
In our investigations, we used an ethanol extract of T. polium.. A recent study (Ljubuncic et al., Citation2005) has shown that a water extract of T. polium. is not cytotoxic for PC12 and HepG2 cell lines. Because the ethanol extract of T. polium. had profound effects on all the cell lines examined in our study, we conclude that the active component(s) of T. polium. can mainly be extracted by ethanol extraction.
The presence of some flavonoids (Harborne et al., Citation1986), diterpenoids (Nagao et al., Citation1982) and selenium (Jurisic et al., Citation2003) in T. polium. may explain its cytotoxicity. In the next step, we are planning to isolate and formulate the different components of T. polium. to recognize the most active component(s) against tumor cells both in vitro. and in vivo..
Acknowledgments
This research was supported by grant no. 82-16-E from the Neuroscience Research Center at Kerman University of Medical Sciences, Kerman, Iran.
References
- Abdollahi M, Karimpour H, Monsef-Esfehani HR (2003): Antinociceptive effects of Teucrium polium. L total extract and essential oil in mouse writhing test. Pharmacol Res 48: 31–35.
- Aggelis G, Athanassopoulos N, Paliogianni A, Komaitis M (1998): Effect of a Teucrium polium. L. extract on the growth and fatty acid composition of Saccharomyces cerevisiae. and Yarrowia lipolytica.. Antonie Van Leeuwenhoek 73: 195–198.
- Autore G, Capasso F, De Fusco R, Fasulo MP, Lembo M, Mascolo N, Menghini A (1984): Antipyretic and antibacterial actions of Teucrium polium. (L.). Pharmacol Res Commun 16: 21–29.
- Baluchnejadmojarad T, Roghani M, Roghani-Dehkordi F (2005): Antinociceptive effect of Teucrium polium. leaf extract in the diabetic rat formalin test. J Ethnopharmacol 97: 207–310.
- Harborne JB, Tomas B, Williams CA, Gil MI, (1986): A chemotaxonomic study of flavonoids from European Teucrium. species. Phytochemistry 25: 2811–2816.
- Jurisic RV-KS, Kalodera Z, Grgic J (2003): Determination of selenium in Teucrium. generation atomic absorption spectrometry. Z Naturforsch [C] 58: 143–145.
- Ljubuncic P, Azaizeh H, Portnaya I, Cogan U, Said O, Saleh KA, Bomzon A (2005): Antioxidant activity and cytotoxicity of eight plants used in traditional Arab medicine in Israel. J Ethnopharmacol 99: 43–47.
- Ljubuncic P, Dakwar S, Portnaya I, Cogan U, Azaizeh H, Bomzon A (2006): Aqueous extracts of Teucrium polium. possess remarkable antioxidant activity in vitro.. Evid Based Complement Alternat Med 3: 329–333.
- Nagao Y, Ito N, Kohno T, Kuroda H, Fujita E (1982): Antitumor activity of Rabdosia. and Teucrium. diterpenoids against P 388 lymphocytic leukemia in mice. Chem Pharm Bull (Tokyo) 30: 727–729.
- Reichinger KH (1982): Flora Iranica. A Kademische Druck und Verlagsanstalt 150: 25–27.
- Stewart BW, Coates AS (2005): Cancer prevention: A global perspective. J Clin Oncol 23: 392–403.
- Tariq M, Ageel AM, al-Yahya MA, Mossa JS, al-Said MS (1989): Anti-inflammatory activity of Teucrium polium.. Int J Tissue React 11: 185–188.