Abstract
Tricholepis glaberrima. DC (Compositae) is popularly known as ‘brahmadandi’ in Ayurveda, the Indian system of medicine. Aerial parts of this plant are used as a nervine tonic, and its infusion was prescribed in seminal debility and impotence. In the current study, we have investigated the effect of methanol extract of the aerial parts in sexually active male rats. In addition, the effect of the extract on the testicular histology and on the activity of two antioxidant enzymes, viz. superoxide dismutase and catalase in testicular homogenate were determined. Administration of the methanol extract at 200 mg/kg body weight for 28 days altered significantly the various components of the sexual behavior study. The extract increased significantly mounting latency (ML) and intromission latency (IL) with a significant reduction in mounting frequency (MF), intromission frequency (IF), and post-ejaculatory interval (PEI). Examination of the testicular histology revealed that the extract favors spermatogenesis by enhancing the proliferation of the seminiferous epithelium. Further, a significant increase in the activity of superoxide dismutase and catalase was observed in rats treated with methanol extract of T. glaberrima.. Therefore, the current findings provide experimental evidence that the extract of T. glaberrima. possesses aphrodisiac properties.
Introduction
Tricholepis glaberrima. DC (Compositae) is a stout, glabrous annual herb popularly known as “brahmadandi” in Ayurveda, the Indian system of medicine (Anonymous, Citation1976; Nadkarni, Citation1976). The plant is hot and bitter, cures “kapha.”, “vata.”, inflammation, and is used in leucoderma and in skin diseases. It is a nervine tonic, aphrodisiac, and is used in seminal debility (Kirtikar & Basu, Citation1975; Nadkarni, Citation1976). A number of Indian medicinal plants have been screened for aphrodisiac properties (Sridhar et al., Citation1993; Subramoniam et al., Citation1997; Ramachandran et al., Citation2004), but there are no reports on aphrodisiac studies with T. glaberrima.. In Ayurvedic texts, aphrodisiacs have been grouped into five categories (Singh & Mukherjee, Citation1998): (1) drugs that stimulate the production of semen, for example, Microstylis wallichii. Lindl (Orchideaceae), Mucuna pruriens. Bak (Leguminosae), Asparagus recemosus. Willd (Liliaceae), and so forth; (2) drugs that improve and purify the quality of semen, for example, Saussurea lappa. Clarke (Compositae), Vetivera ziznoides. Stapf (Gramineae), and so forth; (3) drugs that help sexually and in ejaculation, for example, Strychnos nuxvomica. Linn (Loganiaceae), Myristica fragrans. Houtt (Myristicaceae), and so forth; (4) drugs delaying the time of ejaculation, for example, Sida cordifolia. Linn (Malvaceae), opium, musk, and so forth; and (5) drugs arousing sexual desire, for example, Withania somnifera. Dunal (Solanaceae), Datura stramonium. Linn (Solanaceae), Asparagus recemosus., and so forth.
Considering the lack of scientific studies to support the reported ethnomedicinal uses of T. glaberrima., the current work was designed to evaluate the aphrodisiac properties of the methanol extract of T. glaberrima. and its likely role in the management of seminal debility. Aphrodisiac properties were evaluated by studying the effect of oral administration of the extract on male rat sexual behavior in sexually experienced animals. In addition, effect on the testicular histology and on the activity of antioxidant enzymes was also studied to evaluate its possible role in the management of seminal debility.
Materials and Methods
Plant material and preparation of extract
Aerial parts of T. glaberrima. were obtained from the local market (G. Y. Hakim & Sons Co. Vadodara, Gujarat, India) and were authenticated at the Botany Department of The M.S. University of Baroda, Vadodara, India. A voucher specimen (no. Pharmacy/TG/05-06/02/SP) has been deposited in the Pharmacy Department of The M.S. University of Baroda.
Preparation of extract: Cold maceration method was followed to prepare the methanol extract. In brief, 500 g of the dried aerial parts of the plant material was subjected to maceration with sufficient methanol (95% v/v) for 6 h. The contents were shaken occasionally and then set aside for a period of 18 h after which the extract was filtered. Similar extractions were carried out several times by repeatedly extracting the same marc. Filtrates collected in each extraction were then combined and concentrated on a rotary vacuum evaporator (BUCHI Rotavapor R200, Germany). Vacuum-dried extract (yield = 14% w/w) was used for further studies.
Animals
Adult albino rats of either sex (90 days old) were used for the study. Animals were housed at a temperature of 24–28°C with a relative humidity of 45–55%. Males were placed individually and females in groups with free access to food and water. Before beginning the experiment, the local committee of ethics on animal experimentation approved all experimental procedures (no. 404/01/a/CPCSEA).
Before experimental testing, all the animals were trained for sexual experience. To provide sexual experience, each male rat was allowed 30 min exposure several times to a female rat (used as mating stimulus) in behavioral estrous. Sexually active males, showing ejaculation latency shorter than 15 min in at least the last three sessions, were selected and considered sexually experienced.
Acute toxicity studies
Healthy adult albino rats of either sex, starved overnight, were divided into 4 groups (n = 6) and were fed with increasing doses (250, 500, 1000, and 2000 mg/kg body weight) of the methanol extract. The extract administered orally in doses of up to 2 g/kg body weight did not produce any evident sign of toxicity and any mortality in rats when observed up to 14 days after administration.
Treatment
Sexually active male rats were randomly assigned to one of the following groups (n = 6 each). Group 1 received the vehicle [1 mL of 1% solution of sodium carboxy methyl cellulose (CMC) in water] orally and served as control. Group 2 was administered L-dopa orally (100 mg/kg body weight), and group 3 was administered methanol extract of T. glaberrima. (200 mg/kg body weight) in 1% solution of sodium CMC for 28 days. L-Dopa served as standard (Taglimonte et al., Citation1974; Angrist & Gershon, Citation1976; Anantkumar et al., Citation1994).
Sexual behavior testing protocol
All the sexual behavior tests were conducted 2 h after the onset of darkness. Drugs were administered 1 h before commencement of the experiment. Males were introduced into a rectangular chamber (14 × 14 inch) and a 5-min adaptation period was allowed. Thereafter, each male rat was paired with two stimulus receptive females and sexual behavior was recorded along a period of 1 h (Carro-Juarez et al., Citation2004). Female receptivity was induced by the sequential subcutaneous administration of ethynyl estradiol (10 µg/kg body weight) and hydroxy progesterone caproate (1.5 mg/kg body weight) 48 h and 6 h, respectively, before the copulatory studies. The experiment was conducted in a silent room under dim red light; any jerking movement of the mating area was avoided to enable the rats to chase each other, and clearing of the mating area was done after each trial, as urine trails left by one rat might alter the sexual behavior of the other rat.
The sexual behavior parameters analyzed were mount latency (ML), time from introduction of the female until the first mount; intromission latency (IL), time from introduction of the female until the first mount with pelvic thrusting and vaginal penetration (intromission); mount frequency (MF), number of mounts observed in 60 min; intromission frequency (IF), number of intromissions observed in 60 min; and post-ejaculatory interval (PEI), time from ejaculation until the next intromission. Latency data were expressed in seconds as mean ± SEM and the number of mounts and intromissions as median numbers.
Effect on body weight and organ weight
All the treated and control rats were weighed, and the changes in their body weights were recorded. At the end of treatment, the animals were sacrificed by cervical dislocation and the sexual organs (testis, vas deferens, seminal vesicles and epididymus) were dissected out carefully, freed from adhering tissues, washed in ice-cold saline, blotted, and weighed to the nearest 0.1 mg. The weights of the organs were expressed as mg/100 g of body weight.
Biochemical estimations
Simultaneously, the blood was collected during the sacrifice of animals in the above step in dry centrifuge tubes, which were left at room temperature for 10 min; serum was separated and collected by centrifugation. Serum levels of cholesterol and alkaline phosphatase (ALP) were determined by routine laboratory methods using an autoanalyzer.
Effect on antioxidant enzymes
One testis, obtained as above, from each animal was used for measuring the activity of antioxidant enzymes. A 10% testicular homogenate was prepared using Tris buffer (10 mM; pH 7.4), which was then centrifuged under cold condition (SIGMA 3K30 cooling centrifuge, USA). The clear supernatant was used to determine the activity of the antioxidant enzymes viz. superoxide dismutase (SOD) and catalase (CAT). Superoxide dismutase was estimated by the method of Beauchamp et al. (Citation1971), and catalase was assayed as described by Aebi (Citation1983). Protein content was determined by the method of Lowry et al. (Citation1951).
Histologic preparation and evaluation
Testis from each group was excised quickly during the dissection of animals and was fixed in 10% buffered neutral formalin. Ultrathin sections of the testicular tissue were cut and stained with hematoxylin and eosin. Histologic examinations included the mean seminiferous tubular diameter (STD) and germinal epithelial cell thickness (GECT). Three slides from upper, lower, and mid portions of the testis were prepared and evaluated for each testis. The mean STD and GECT were calculated in 20 seminiferous tubules using a projection microscope (Ulusoy et al., Citation2004) and were expressed in micrometers. Photomicrographs were recorded in 10 × magnifications.
Data analysis
To determine statistically significant differences among treatments, the sexual behavior data were analyzed by means of Student's t.-test and one-way ANOVA followed by Dunnett tests. Latency results were expressed as mean ± SEM and numbers as medians. Values of p < 0.05 were considered statistically significant.
Results
Effect of extract on sexual behavior of the rats
In the first part of the current study, copulatory behavior was examined in sexually experienced male rats that were subacutely treated with 200 mg/kg body weight of the methanol extract of T. glaberrima. and the results were compared with control (vehicle treated) and standard (L-dopa). Results of the coupulatory behavior study () show that the methanol extract of T. glaberrima. at the dose of 200 mg/kg body weight significantly increased the MF and IF and caused a significant reduction in ML and IL. All these effects were observed on the 7th, 14th, 21st, and 28th days. PEI was significantly altered on the 28th day in both standard and test groups.
Table 1.. Effect of oral administration of T. glaberrima. methanol extract on copulatory behavior in male rats.
Effect on body weight and organ weight
A significant increase in the body weight was observed in all the groups, but the increase was found to be much higher in T. glaberrima.–treated groups compared with control (). Treatment with T. glaberrima. showed a significant increase in the weight of seminal vesicles, whereas only a greater increase in the weight of other reproductive organs was observed compared with control group ().
Table 2.. Changes in the body weight and reproductive organs of rats after T. glaberrima. treatment.
Effect on biochemical parameters
A significant decrease in the serum cholesterol was observed in animals treated with L-dopa and methanol extract of T. glaberrima. and a significant increase in the serum ALP was observed in rats treated with L-dopa and methanol extract of T. glaberrima. ().
Table 3.. Effect on biochemical parameters and antioxidant enzymes after treatment with T. glaberrima..
Effect on the antioxidant enzymes
Effect of extract on the activity of antioxidant enzymes was measured in testicular homogenate. Animals treated with L-dopa and methanol extract of T. glaberrima. showed a significant increase in SOD and CAT activity compared with control group ().
Effect on testicular histomorphology
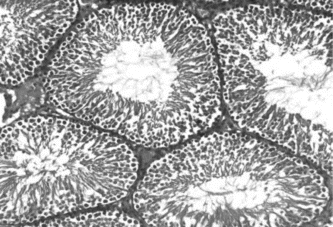
, , and show the histology of the testis in rats treated with vehicle, L-dopa, and T. glaberrima., respectively. shows the mean values of the STD and GECT of the testis in all the three groups. From the results, it was observed that the values of mean STD and GECT were significantly higher in T. glaberrima. and L-dopa treated groups compared with control.
Figure 1 Histology of the testis in a rat treated with vehicle showing the normal seminiferous tubular diameter and germinal epithelial cell thickness.
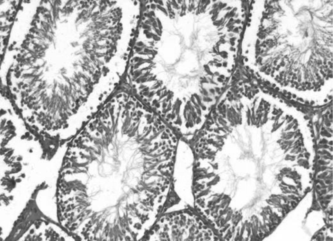
Figure 3 Histology of the testis in a rat treated with methanol extract of T.glaberrima. showing the better proliferation of seminiferous tubules with a marked increase in germinal epithelium.
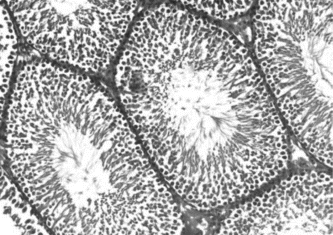
Table 4.. Effect of T. glaberrima. on testicular histomorphology.
Discussion
The current study provides evidence on the ability of the plant extract to enhance male sexual behavior in sexually active rats. The data obtained reveal that when administered orally, methanol extract of T. glaberrima. effectively facilitates several components of the copulatory behavior. The plant is reported to be used as a nervine tonic, and such drugs enhance the blood circulation and produce general well-being by toning up the mental and physical functions. Therefore, the observed activity could be due to either drug-induced changes in neurotransmitter levels or their action in the cells. The increase in the body weight of T. glaberrima.–treated rats could be due to the androgenic properties of this plant as androgens possess anabolic activity (Johnson & Everitt, 1988).
Cholesterol is known to be a precursor in androgen biosynthesis, and its level is closely related to the fertility and the sperm output (Neena et al., Citation1987). Increase in testicular phospholipids and cholesterol may be due to decrease in testes function or viceversa. In the current findings, decreased level of cholesterol may be due to its increased utilization for steroidogenesis, which may be either by pituitary stimulation or by a direct stimulatory action on target tissue, and this decreased level is suggestive of an increased androgen level. A significant increase in the alkaline phosphatase level was observed in the animals treated with T. glaberrima.. The enzyme ALP was found to increase the prostatic secretions and helps to maintain the appropriate pH of seminal fluid, which in turn is necessary for retaining the sperm motility (Lucchetta et al., Citation1984; Clavert et al., Citation1986; Carani et al., Citation1991). Thus, the plant T. glaberrima. may be an effective therapy for male infertility, especially in cases of diminished prostatic secretions.
Reactive oxygen metabolites, such as hydrogen peroxide, superoxide radical, nitric oxide radical, and so forth, appear to play many diverse roles in the maintenance and disruption of cell physiology. On the one hand, they have been found to perform cell signaling functions in different cell types, including spermatozoa, and on the other hand they have been linked to ageing, disease, and apoptosis (Koshio et al., Citation1988; Sundquivst, Citation1991; Yaki, Citation1993; Aitken et al., Citation1995). The role of oxidative stress in the etiology of male infertility has been clearly established by a series of studies (Sanocka et al., Citation1996; Aitken et al., Citation1998; Twigg et al., Citation1998). High concentration of these reactive oxygen species (ROS) is associated with loss of sperm motility and decreased capacity for sperm oocyte fusion (Aitken et al., Citation1989), thus making the health of the sperm to depend upon the antioxidants. From the results, it is observed that there is a significant increase in the activity of superoxide dismutase and catalase, the two powerful antioxidant enzymes of the body, in the testicular homogenate of the animals treated with T. glaberrima. when compared with that in control animals. These enzymes may help to retain the motility and viability of the sperm by effectively neutralizing the deleterious effects of the ROS and thereby may help in improving the success rate of treating male infertility.
Studies on the testicular histomorphology showed that mean STD and GECT were significantly higher in the animals treated with T. glaberrima. compared with control group. Increase in these values is an indication of better proliferation of the testicular tissues and thereby representing better spermatozoal maturation within the seminiferous tubules leading to healthier spermatogenesis.
The chemical constituents and the mechanism of action responsible for these activities are not known. However, the plant has been reported to contain phytosterols (spinasterol, stigmasterol), terpenoids, flavonoids (Chawla et al., Citation1976; Manerikar et al., Citation1978), and so forth. The flavonoid content of the plant may also contribute to its sex-stimulating activity as flavonoids were shown to alter the androgen levels (Ageel et al., Citation1994), which play an important role in sexual stimulation, and also it is well-known that these compounds increase significantly SOD and catalase activities (Toyokuni et al., Citation2003) thereby imparting an indirect potentiating effect on the observed activity.
In conclusion, the results presented in this article indicate the potential value of T. glaberrima. as a therapeutic agent in treating male infertility. The investigation shows that the methanol extract of T. glaberrima. can enhance the sexual activity in normal rats and favors spermatogenesis by enhancing the proliferation of seminiferous epithelium. As mentioned in the “Introduction”, plants show aphrodisiac properties by various mechanisms and from the data obtained in the current study, the plant T. glaberrima. was found to fall under the category of aphrodisiacs that increase the sexual desire and those that improve the quality and stimulate the production of semen. Generally, sexual behaviors are enhanced by elevated neurotransmitter levels or by direct action on the target tissues, which may be due to one of the factors discussed here. Investigations are in progress in this laboratory to explore the possible site(s) and mechanism(s) of action involved in the effects observed from the methanol extract of T. glaberrima..
Acknowledgments
One of the authors (S.A.P.) would like to thank the All India Council for Technical Education (AICTE), New Delhi, for providing financial assistance in the form of a National Doctoral Fellowship (NDF) to carry out this work.
References
- Aebi HE (1983): Methods of Enzymatic Analysis, 3rd ed., Vol. 3. Deerfield Beach, FL, Verlag Chemie, p. 273.
- Ageel AM, Islam MW, Ginawi OT, Al-Yahya MA (1994): Evaluation of the aphrodisiac activity of Listea chinensis. (Lauraceae) and Orchis malculata. (Orcidaceae) extracts in rats. Phytother Res 3: 103–105.
- Aitken RJ, Clarkson JS, Fishel S (1989): Generation of reactive oxygen species, lipid peroxidation and human sperm function. Biol Reprod 40: 183–197.
- Aitken RJ, Gordan E, Harkiss D, Twig JP, Milne P, Jennings Z, Irvine DS (1998): Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod 59: 1037–1046.
- Aitken RJ, Paterson M, Fisher H, Buckingham DW, Duin MV (1995): Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci 108: 2017–2025.
- Ananthakumar KV, Srinivasan KK, Shanbhag T, Rao SG (1994): Aphrodisiac activity of the seeds of Mucuna pruriens.. Indian Drugs 31: 321–327.
- Angrist B, Gershon S (1976): Clinical effects of amphetamine and L-dopa on sexuality and aggression. Compr Psychiatry 17: 715–722.
- Anonymous (1976): The Wealth of India, Raw Materials, Vol. 10. New Delhi, CSIR, p. 286.
- Beauchamp C, Fridovich I (1971): Superoxide dismutase: Improved assays and assay applicable to acrylamide gels. Anal Biochem 44: 276–287.
- Carani C, Salvioli C, Scuteri A (1991): Urological and sexual evaluation of treatment of benign prostatic disease using Pygeum africanum. at high doses. Arch Ital Urol Nefrol Androl 63: 341–345.
- Carro-Juarez M, Cervantes E, Cervantes-Mendez M, Rodriguez-Manzo G (2004): Aphrodisiac properties of Montanoa tomentosa. aqueous crude extract in male rats. Pharmacol Biochem Behav 78: 129–134.
- Chawla AS, Kapoor VK, Sangal PK, Gupta AK, Evans FJ (1976): Chemical constituents of Tricholepis glaberrima.. Planta Med 30: 151–153.
- Clavert A, Cranz C, Riffaud JP (1986): Effects of an extract of the bark of Pygeum africanum. on prostatic secretions in the rat and in man. Ann Urol 20: 341–343.
- Johnson MH, Everitt BJ (1998): Essential Reproduction. Great Britain, Blackwell Scientific Publications, p. 134.
- Kirtikar KR, Basu BD (1975): Indian Medicinal Plants, Vol. 2. Delhi, Periodical Experts, p. 1425.
- Koshio O, Akanuma Y, Kasuga M (1988): Hydrogen peroxide stimulates tyrosine phosphorylation of the insulin receptor and its tyrosine kinase activity in intact cells. Biochem J 250: 95–101.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951): Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275.
- Lucchetta G, Weill A, Becker N (1984): Reactivation of the secretion from the prostatic gland in cases of reduced fertility. Biological study of seminal fluid modifications. Urol Int 39: 222–224.
- Manerikar SV, Kulkarni AB (1978): Chemical investigation of Tricholepis glaberrima.. Indian J Chem S B 16: 439–440.
- Nadkarni AK (1976): Indian Materia Medica, Vol. 1. Bombay Popular Prakashan Pvt. Ltd., p. 1234.
- Neena N, Edwards MS, Bedwal RS, Mathur RS (1987): Effect of adrenalectomy and adrenalactomy-hydrocortisone treatment on zinc, biochemical parameters and histology of testes of rats. Indian J Exp Biol 25: 651–659.
- Ramachandran S, Sridhar Y, Kishore GS, Saravanan M, Thomas LJ, Anbalagan N, Sridhar SK (2004): Aphrodisiac activity of Butea frondosa. extract in male rats. Phytomedicine 11: 165–168.
- Sanocka D, Miesel R, Jedrzejczak P, Kurpisz MK (1996): Oxidative stress and male infertility. J Androl 17: 449–454.
- Singh G, Mukherjee T (1998): Herbal aphrodisiacs: A review. Indian Drugs 35: 175–182.
- Sridhar M, Srinivasan KK, Shanbhag T, Gurumadhavarao S (1993): Aphrodisiac activity of Ipomea digitata.. Indian Drugs 30: 165–169.
- Subramoniam S, Madhavachandran V, Rajashekharan S, Pushpangadan P (1997): Aphrodisiac properties of Trichopus zeylanicus. extract in male mice. J Ethnopharmacol 57: 21–27.
- Sundquivst T (1991): Bovine aortic endothelial cells release hydrogen peroxide. J Cell Physiol 148: 152–156.
- Taglimonte A, Fratta W, Gessa GL (1974): Aphrodisiac effect of L-dopa and apomorphine in male sexually sluggish rats. Experentia 30: 381–382.
- Toyokuni S, Tanaka T, Kawaguchi W, Fang NR, Ozeki M, Akatsuka S, Hiai H, Okezie I, Bahorun T (2003): Effect of the phenolic contents of Mauritian endemic plant extract on promoter activities of antioxidant enzymes. Free Radic Res 37: 1215–1224.
- Twigg J, Fulton N, Gomez E, Irvine DS, Aitken RJ (1998): Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: Lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum Reprod 13: 1429–1436.
- Ulusoy E, Cayan S, Yilmaz N, Aktas S, Acar D, Doruk E (2004): Interferon α-2B may impair testicular histology including spermatogenesis in a rat model. Arch Androl 50: 379–385.
- Yaki K (1993): Lipid peroxides free radicals and disease. In: Yaki K, ed., Active Oxygens, Lipid Peroxides and Antioxidants. Boca Raton, FL, CRC Press, pp. 1–38.