Abstract
This research collected 27 Lauraceae tree species in Taiwan, and the extracts prepared from leaves and branches were selected to evaluate and characterize their putative bioactivities and potential medicinal applications. Several bioactivity assays, including antifungal tests, antioxidant evaluation, anti-inflammation activity, and cytotoxicity were preformed in this study.The results showed no significant antifungal activity by Lauraceae extracts. Neolitsea parvigemma. (Hay.) Kanehira et Sasaki expresses the best antioxidant activity (IC50 = 5.73 µg/mL) in the DPPH assay.The extracts of Litsea akoensis. Hay. and Cryptocarya concinna. Hance had significant anti-inflammation activity, and they can inhibit the nitric oxide (NO) production in the LPS-induced microphage assay at the dose of 25 µg/mL. According to the cytotoxicity assay, Lindera aggregate. (Sims) Kosterm and Cryptocarya concinna. Hance extracts showed in vitro. cytotoxicity against human umbilical vein endothelial cell line (HUVEC) with IC50 values of 43.15 µg/mL and 49.36 µg/mL, respectively, and Phoebe formosana. (Matsum. et Hay.) Hay. extract exhibited marked cytotoxicity (IC50 = 42.87 µg/mL) against a human leukemia cell line (HL-60). Results from this preliminary investigation suggest that these Lauraceae tree species may have a great potential for further development as cancer chemoprevention agents or food supplements for promoting human health.
Introduction
There are more than 2500 species belonging to the Lauraceae family all over the world, distributed within the subtropics and tropics of eastern Asia and South and North America (Simie et al., Citation2004). Many plants of Lauraceae have been employed in folk medicine for their interesting bioactivities. For example, Cinnamomum camphora. (L.) Presl is a major source of camphor, which can be made into camphor oil and mothballs. In addition, camphor is taken orally to calm hysteria, nervousness, neuralgia, and to treat serious diarrhea. Camphor is also known to be effective in treating colds and chills (Lee et al., Citation2006). The bark of Cinnamomum cassia. Blume is a very famous traditional medicine that has been widely used in Asian countries. The extracts from C. cassia. have been claimed to reduce inflammation (Lee & Shibamoto, Citation2002), and to decrease serum glucose, total cholesterol, and platelet counts (Khan et al., Citation2003).
Owing to its unique ecosystem, Taiwan is famous for the abundance and diversity of its flora, with more than 4500 plant species classified to date. In Taiwan, Lauraceae is an economically important family, consisting mostly of trees, and growing throughout the island, from the lowlands up to an altitude of 1500 m (Liao, Citation1996). There are around 60 Lauraceae tree species grown in Taiwan. Although there are some studies that focus on bioactivity investigations of Lauraceae grown in Taiwan (), systematic collection and bioactivities screening are still worthy of further investigation. On the other hand, from a natural conservation point of view, the most environment-friendly strategy is not to utilize the entire tree, but to utilize its twigs and/or leaves. In our current study, the twigs and leaves from 27 tree species of Lauraceae grown in Taiwan were collected. Several bioassays, including antifungal activity, antioxidant activity, anti-inflammation activity, and cytotoxicity, were performed to evaluate potential bioactivity.
Table 1.. Lauraceae plants collected in this study and reported bioactivity.
Materials and Methods
Chemicals and reagents
1,1-Diphenyl-2-picrylhydrazyl (DPPH), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), vitamin C, and lipopolysaccharide (LPS) were purchased from Sigma (St. Louis, MO, USA). All other chemicals and solvents used in this study were of reagent or HPLC grade.
Plant and extract preparations
All of samples used in this study were collected from the Experimental Forest of National Taiwan University and Liukuei Education Center of Taiwan Forestry Research Institute in 2005 (). The samples were identified by Prof. Y.-H. Tseng (Department of Forestry, National Chung-Hsing University). Voucher specimens were deposited in the herbarium of the Department of Forestry, NCHU. The extracts were prepared by the following procedure. Fresh leaf and twig mixture (500 g) was extracted twice with 2.5 L of methanol at ambient temperature. The extracts were decanted, filtered under vacuum, concentrated in a rotary evaporator, and then lyophilized. The resulting powder extracts were employed for the current study.
Antifungal assay
The fungi used were Trametes versicolor. (BCRC 35253) and Laetiporus sulphureus. (BCRC 35305). In vitro. antifungal assays were performed as in our previous study (Chang et al., Citation1999). Assays were carried out in triplicate, and data were averaged. Extracts (100 µg/mL) were added to sterilized potato dextrose agar (PDA). The testing Petri dishes were incubated in the dark at 26 ± 2°C and 70% relative humidity. When the mycelium of fungi reached the edges of the control Petri dishes, the antifungal indices were calculated. Each test was repeated three-times, and the data were averaged. The antifungal index was calculated as follows:
Free radical scavenging activity
The scavenging activity for DPPH radicals by plant extracts from Lauraceae was measured according to the method as described previously (Chang et al., Citation2001b; Wang et al., Citation2002). Assays were performed in 300 µL reaction mixtures, containing 200 µL of 0.1 mM DPPH-ethanol solution, 90 µL of 50 mM Tris-HCl buffer (pH 7.4), and 10 µL of ethanol (as solvent blank) or test plant extracts and ascorbic acid were used as positive controls. After 30 min of incubation at room temperature, absorbance (540 nm) of the reaction mixtures was taken by ELISA reader (μQuant, Bio-Tek Instruments, Winooski, VT, USA). The inhibitory effect of DPPH was calculated according to the following formula:
IC50 represents the levels at which 50% of the radicals were scavenged by test samples.
Nitric oxide inhibition assay
Nitric oxide (NO) inhibition activities of Lauraceae extracts were conducted according to the method used previously (Wang et al., Citation2003). RAW 264.7 cells, a murine macrophage cell line, were obtained from ATCC (Rockville, MD, USA) and cultured at 37°C in Dulbecco's modified essential medium supplemented with 10% FBS, 100 units/mL penicillin, and 100 µg/mL streptomycin in a 5% CO2 incubator, as recommended by ATCC. RAW 264.7 cells grown in T75 culture flasks were harvested and seeded in 96-well plates at a density of 2 × 105 cells/well. Adhered cells were incubated for 24 h with (positive control) or without (negative control) 1 µg/mL LPS, in the absence or presence of test extracts. Nitrite concentration, as a parameter of NO synthesis, in the culture supernatant of RAW 264.7 cells was measured by the Griess reaction (Schmidt & Kelm, Citation1996). Briefly, 100 µL cell culture supernatants were reacted with 100 µL of Griess reagent [1:1 mixture of 0.1% N.-(1-naphthyl)ethylenediamine in H2O and 1% sulfanilamide in 5% phosphoric acid] in a 96-well plate, and absorbance was recorded using an ELISA reader (μQuant) at 540 nm. Results were expressed as a percentage of inhibition relative to the control (cell treated with LPS alone). In parallel to the Griess assays, RAW 264.7 cells treated with or without the extracts were tested for cell viability using the MTT (4,5-dimethylthiazol-2-yl-2,5,-diphenyltetrazolium bromide) based colorimetric assay (Scudiero et al., Citation1988).
Tumor cell growth inhibition assay
Cytotoxicity was performed using a MTT assay (Alley et al., Citation1988; Song et al., Citation1994; Chang et al., Citation2000). Tumor cells [human tumor cells including HUVEC (human umbilical vein endothelial cell), MCF-7 (breast adenocarcinoma), and HL-60 (human leukemia)] (1 × 105 cells/mL) were seeded into a 96-well plate in triplicate and preincubated for 12 h in order to perform cell attachment. Then, 100 µL fresh medium containing various concentrations (500, 250, 100, 50, and 25 µg/mL of methanol extracts) of test compound were added into the 96-well plate. The cells were incubated with each compound at 37°C for 24 h under humidified air containing 5% CO2. Cell survival was evaluated by adding 100 µL tetrazolium salt solution (1 mg MTT/mL in PBS). After 4 h of incubation at 37°C, 100 µL DMSO was added to dissolve the precipitates of reduced MTT. Microplates were then shaken for 15 min, and the absorbance was determined at 570 nm in a multiwell scanning spectrophotometer.
Results and Discussion
Antifungal activity of methanol extracts from Lauraceae
To evaluate the antifungal activities of extracts from Lauraceae against fungi, we selected two representative fungi, T. versicolor. (white-rot fungus) and L. sulphureus. (brown-rot fungus) as testing strains. According to the results obtained from antifungal assays, the antifungal indices were lower than 10.0% against both fungi at the dosage of 100 µg/mL (data not show), indicating the extracts of Lauraceae examined in this study did not show a significant antifungal activity. Wang and his co-workers (Citation2005) have demonstrated that the essential oils of C. osmophloeum. possessed strong antifungal activity. Surprisingly, the methanol extracts of C. osmophloeum. did not show the expected antifungal activity in this study. It might be due to the low amount of active component (cinnamaldehyde) in the methanol extracts of C. osmophloeum. (Wang et al., Citation2005). Overall, the antifungal performance of Lauraceae tree species studied herein was not considerable.
Radical scavenging activities of methanol extracts from Lauraceae
The extracts from Lauraceae tree species were tested for their capacity to scavenge free radicals of DPPH, which has been used to evaluate the antioxidant activity of natural products from plants globally (Wang et al., Citation2002). The results of DPPH scavenging activities are shown in . Most extracts from Lauraceae revealed good scavenging activities for DPPH radicals. The EC50 of four species, including C. subarenium. (EC50 = 6.12 µg/mL), L. acuminata. (EC50 = 6.85 µg/mL), N. parvigemma. (EC50 = 5.73 µg/mL), and N. variabillima. (EC50 = 7.41 µg/mL), were lower than 10 µg/mL. In comparison with well-known antioxidants, ascorbic acid (EC50 = 1.5 µg/mL) and quercetin (EC50 = 2.3 µg/mL), the crude extracts of the trees mentioned above exhibited good antioxidant activity. Reactive oxygen species (ROS) are essential for life for they are involved in cell physiology. However, over production of ROS is suggested to be strongly associated with the aging process and certain degenerative diseases including various cancers, cognitive dysfunctions, and coronary heart disease (Finkle & Holbrook, Citation2000). Thus, it is important to discover effective antioxidants from natural sources, especially from plant species, to reduce ROS activities. On the basis of the study using in vitro. DPPH radical scavenging assay, we suggest that Lauraceae plants, such as C. subarenium., L. acuminata., N. parvigemma., and N. variabillima., are potential candidates to serve as supplements for human health care.
Table 2.. DPPH free radical scavenging activities of extracts from 27 Lauraceae tree spicees.
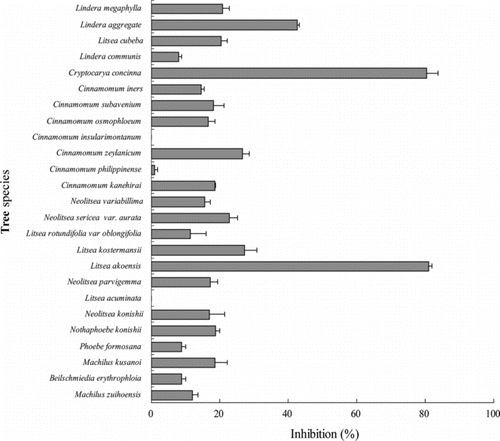
Inhibition of nitric oxide production in LPS-stimulated RAW 264.7 cells
Activation of macrophages plays a critical role in the inflammatory process by releasing a variety of inflammatory mediators (Zhuang et al., Citation1998), such as NO, which is a critical signaling molecule produced at inflammatory sites by inductible nitric oxide synthase (iNOS), which is often expressed in response to LPS and a variety of proinflammatory cytokines (MacMicking et al., Citation1997). In this study, the effects of methanol extracts from Lauraceae on NO synthesis in RAW 264.7 macrophages were investigated. As shown in , Lauraceae methanol extracts exhibited significant inhibition of nitrite production. Among the test extracts, Litsea akoensis. and Cryptocarya concinna. extracts exhibited the most significant inhibitory activity; 81.07% and 80.37% of NO production was inhibited at the dose of 25 µg/mL, respectively.
Tumor cell growth inhibition assay
Cytotoxicity of Lauraceae extracts was evaluated by a MTT assay, which measures the relative metabolic rate activity of the cells (Alley et al., Citation1988; Song et al., Citation1994). The cytotoxicity of the methanol extracts from Lauraceae was tested against HUVEC, MCF-7, and HL-60 cell lines in this study. As shown in , Lindera aggregate. (IC50 = 43.15 µg/mL), Cryptocarya concinna. (IC50 = 49.36 µg/mL), and Phoebe formosana. (IC50 = 42.87 µg/mL) showed significant cytotoxicity for HUVEC and HL-60, respectively. However, the methanol extracts did not display any cytotoxicity against the MCF-7 cancer cell line. On the basis of the results obtained, effective antitumor active compounds from the methanol extracts of Lindera aggregate., Cryptocarya concinna., and Phoebe formosana. can be obtained when further separation and purification are carried out in the near future.
Table 3.. Cytotoxicity activity of 15 tree species extracts against HUVEC, HL-60, and MCF-7 cells.
Conclusions
The extracts from 27 woody plants of Lauraceae grown in Taiwan were assayed to explore their bioactivities. The results indicated that a number of extracts present significant activities, such as antioxidant, anti-inflammation, antitumor activities. This study provides valuable and useful information and indications for further exploring the potential nutraceutical and pharmaceutical applications of the Lauraceae tree species. Further investigations will be conducted by our research team.
References
- Alley MC, Scudiero DA, Monks A, Hursey ML, Ciezerwinski MJ, Fine DL (1988): Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 48: 589–601.
- Atta AH, Alkofahi A (1998): Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol 60: 117–124.
- Chao LK, Hua KF, Hsu HY, Cheng SS, Liu JY, Chang ST (2005): Study on the antiinflammatory activity of essential oil from leaves of Cinnamomum osmophloeum.. J Agric Food Chem 53: 7274–7278.
- Chang ST, Wang SY, Wu CL, Su YC, Kuo YH (1999): Antifungal compounds in the ethyl acetate soluble fraction of the extractives of Taiwania (Taiwan cryptomerioides. Hayata) heartwood. Holzforschung 53: 487–490.
- Chang ST, Wang SY, Wu CL, Shiah SG, Kuo YH, Chang CJ (2000): Cytotoxicity of extractives from Taiwania cryptomerioides. heartwood. Phytochemistry 55: 227–232.
- Chang ST, Chen PF, Chang SC (2001a): Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum.. J Ethnopharmacol 77: 123–127.
- Chang ST, Wu JH, Wang SY, Kang PL, Yang NS, Shyur LF (2001b): Antioxidant activity of extracts from Acacia confuse. bark and heartwood. J Agric Food Chem. 49: 3420–3424.
- Chen IS, Lai-Yaun IL, Duh GY, Tsai IL (1998): Cytotoxic butanolides from Litsea akoensis.. Phytochemistry 49: 745–750.
- Chen KS, Hsieh PW, Hwang TL, Chang FR, Wu YC (2005): Anti-inflammatory furanogermacrane sesquiterpenes from Neolitsea parvigemma.. Nat Prod Res 19: 283–286.
- Chen PF, Chang ST, Wu HH (2002): Antimite activity of essential oils and their components from Cinnamomum osmophloeum. (in Chinese with English abstract). Quarterly J Chinese Forestry 35: 397–404.
- Cheng MJ, Tsai IL, Lee SJ, Jayaprakasam B, Chen IS (2005): Steryl epoxide, secobutanolide and butanolides from the stem wood of Machilus zuihoensis.. Phytochemistry 66: 1180–1185.
- Choi EM, Hwang JK (2004): Effects of methanol extract and fractions from Litsea cubeba. bark on the production of inflammatory mediators in RAW264.7 cells. Fitoterapia 75: 141–148.
- Fang SH, Rao YK, Tzeng YM (2004): Cytotoxic effect of trans.-cinnamaldehyde from Cinnamomum osmophloeum. leaves on human cancer cell lines. Int J Appl Sci Eng 2: 136–147.
- Finkle T, Holbrook NJ (2000): Oxidants, oxidative stress and the biology of aging. Nature 408: 239–247.
- Furuno T, Yasuto T, Shoichi Y, Tohru U, Susumu J (1994): Activities of leaf oils and their components from Lauraceae tree against house dust mites. Mokuzai Gakkaishi 40: 78–87.
- Hou WC, Lin RD, Cheng KT, Hung YT, Cho CH, Chen CH, Hwang SY, Lee MH (2003): Free radical-scavenging activity of Taiwanese native plants. Phytomedicine 10: 170–175.
- Huang RL, Chen CC, Huang YL, Ou JC, Hu CP, Chen CF, Chang C (1998): Anti-tumor effects of d-dicentrine from the root of Lindera megaphylla.. Planta Med 64: 212–215.
- Hwang JK, Choi EM, Lee JH (2005): Antioxidant activity of Litsea cubeba.. Fitoterapia 76: 684–686.
- Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK (2003): Volatile constituents from Cinnamomum zeylanicum. fruit stalks and their antioxidant activities. J Agric Food Chem. 51: 4344–4348.
- Khan A, Safdar M, Ali-Khan MM, Khattak KN, Anderson RA (2003): Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 26: 3215–3218.
- Kim NY, Ryu JH (2003): Butanolides from Machilus thunbergii. and their inhibitory activity on nitric oxide synthesis in activated macrophages. Phytother Res. 17: 372–375.
- Lee HJ, Hyuna EA, Yoon WJ, Kim BH, Rhee MH, Kang HK, Cho JY, Yoo ES (2006): In vitro. anti-inflammatory and anti-oxidative effects of Cinnamomum camphora. extracts. J Ethnopharmacol 103: 208–216.
- Lee KG, Shibamoto T (2002): Determination of antioxidant potential of volatile extracts isolated from various herbs and spices. J Agric Food Chem. 50: 4947–4952.
- Liao JC (1996): Lauraceae. In: Editorial Committee of the Flora of Taiwan (eds.), Flora of Taiwan II, 2nd ed., National Taiwan University, Taipei, Taiwan, pp. 433–485.
- Lin CC, Cheng HY, Fang BJ (2003): Anti-Herpes virus type 2 activity of herbal medicines from Taiwan. Pharm Biol 41: 259–262.
- MacMicking J, Xie QW, Nathan C (1997): Nitric oxide and macrophage function. Annu Rev Immunol 15: 323–350.
- Mori A, Yokoi I, Noda Y, Willmore LJ (2004): Natural antioxidants may prevent posttraumatic epilepsy: A proposal based on experimental animal studies. Acta Medica Okayama 58: 111–118.
- Schmidt HHHW, Kelm (1996): Determination of nitrite and nitrate by the Griess reaction. In: Feelisch M, Stamler, JS, eds., Methods in Nitric Oxide Research. New york, Wiley, pp. 491–497.
- Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR (1988): Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res 48: 4827–4833.
- Simie A, Sokovie MD, Ristic M, Grujic-Jovanovic S, Vukojevic J, Marin PD (2004): The chemical composition of some Lauraceae essential oils and their antifungal activities. Phytother Res 18: 713–717.
- Song YN, Zhang HL, Chang CJ (1994): Cytotoxic cyclolignans from Koelreuteria henryi.. J Nat Prod 57: 1670–1674.
- Su MJ, Chen WP, Lo TY, Wu TS (1999): Ionic mechanisms for the antiarrhythmic action of cinnamophilin in rat heart. J Biomed Sci 6: 376–386.
- Tsai IL, Hung CH, Duh CY, Chen IS (2002): Cytotoxic butanolides and secobutanolides from the stem wood of formosan Lindera communis.. Planta Med 68: 142–145.
- Wang SY, Kuo YH, Chang HN, Kang PL, Tasy HS, Lin KF, Yang NS, Shyur LF (2002): Profiling and characterization antioxidant activities in Anoectochilus formasanus. Hayata. J Agric Food Chem 50: 1859–1865.
- Wang SY, Chang HN, Lin KT, Lo CP, Yang NS, Shyur LF (2003): Antioxidant properties and phytochemical characteristics of extracts from Lactuca indica.. J Agric Food Chem. 51: 1506–1512.
- Wang SY, Chen PF, Chang ST (2005): Antifungal activities of essential oils from indigenous cinnamon (Cinnamomum osmophloeum.) leaves. Bioresource Technol. 96: 813–818.
- Yan XH, Wei XY, Xie HH, Liu MF, Zhang FX (2000): Aporphine alkaloids of Litsea rotundifolia. and L. rotundifolia. var. oblongifolia.. J Trop Subtrop Bot 8: 324–328.
- Yu SM, Hsu SY, Ko FN, Chen CC, Huang YL, Huang TF, Teng CM (1992): Haemodynamic effects of dicentrine, a novel alpha 1-adrenoceptor antagonist: Comparison with prazosin in spontaneously hypertensive and normotensive Wistar-Kyoto rats. Br J Pharmacol 106: 797–801.
- Yu SM (1994): Thaliporphine selectively inhibits expression of the inducible, but not the constitutive, nitric oxide synthase. Biochem J 303: 289–294.
- Zhuang JC, Lin C, Lin D, Wogan GN (1998): Mutagenesis associated with nitric oxide production in macrophages. Proc Natl Acad Sci USA 95: 8286–8291.