Abstract
Historically, the fruit of Tribulus terrestris. Linn. (Zygophyllaceae) has been used in India and China as a constituent of rejuvenation tonics. It is also used in traditional Indian medicine in the therapy of a variety of health conditions affecting liver, kidney, and cardiovascular and immune systems. Oxidative stress has been implicated in some of these disease conditions. Therefore, we have investigated the antioxidant activity of the aqueous extract of Tribulus terrestris. fruit (TTE) in spleen cells. Generation of intracellular reactive oxygen species (ROS) in response to γ-radiation and 2,2′-azobis.(2-propionimidinedihydrochloride) (AAPH) was measured by flow cytometry using dichlorodihydrofluorescein diacetate (H2DCFDA). TTE scavenged ROS induced by γ-radiation as well as AAPH in a concentration-dependent manner. TTE also showed protection against oxidative stress–induced apoptosis. In addition, TTE exhibited mitogenic activity in the spleen cells. We conclude that TTE attenuates oxidative stress and protects cells from lethal oxidant damage, and thus its therapeutic potential in treating many ailments may relate to its antioxidant properties.
Introduction
Tribulus terrestris. Linn. (Zygophyllaceae) (puncture vine) is widely distributed in the subtropical areas around the world. The fruit of T. terrestris. is commonly known as “gokhuru” or “jili” in traditional Indian and Chinese medicine, respectively. It has been commonly used in folk medicine as a diuretic and in treatment of colicky pains, hypertension, and hypercholesterolemia (Al-Ali et al., Citation2003; Sharifi et al., Citation2003; Phillips et al., Citation2006). It is popularly claimed to improve sexual function possibly through increase in the free serum testosterone (Brown et al., Citation2001) and augmentation of nicotinamide adenine dinucleotide phosphate–diaphorase activity and androgen receptor expression (Gauthaman & Adaikan, Citation2005). Anthelmintic (Deepak et al., Citation2002), antifungal (Bedir et al., Citation2002; Zhang et al., Citation2006), and antibacterial (Ali et al., Citation2001) activities have also been reported in T. terrestris. extracts. Steroidal saponins from T. terrestris. have been reported to possess anticancer activity (Bedir et al., Citation2002; Kumar et al., Citation2006) and to protect hepatocytes from cell death (Li et al., Citation1998).
The cellular redox state is being increasingly recognized as a crucial mediator of multiple metabolic, signaling, and transcriptional processes in cells, essential for their normal functioning and survival (Ichiki et al., Citation2003; Williams & Kwon, Citation2004; Nakashima et al., Citation2005). Reactive oxygen species (ROS) are continuously generated endogenously during the cellular metabolic processes and they are counteracted by the intracellular antioxidant defenses. Unregulated production of ROS, leading to oxidative stress, has been implicated in a growing list of clinical disorders such as atherosclerosis, diabetes, rheumatoid arthritis, cancer, stroke, cataract, liver, and prostate disorders (Rice-Evans & Diplock, Citation1992; Ozben, Citation1998; Valko et al., Citation2004). Mechanisms responsible for the ROS-mediated injury to cells and tissues include lipid peroxidation, oxidative DNA damage, and protein oxidation (Halliwell & Gutteridge, Citation1989), and also cell death (Buttke & Sandstrom, Citation1994; Matsuzawa & Ichijo, Citation2005). Natural antioxidant mechanisms have been found to be defective in these diseases. In many parts of the world, indigenous plants are used in traditional therapies of diseases in which oxidative stress is implicated, for example, cerebral damage, diabetes, liver disease, and jaundice (Ichikawa & Konishi, Citation2002; Kim et al., Citation2004; Ljubuncic et al., Citation2005; Margaill et al., Citation2005).
Despite the widespread usage of T. terrestris. in the traditional medicinal system, the mechanism of its therapeutic efficacy is not known. We have, therefore, investigated the antioxidant activity of the T. terrestris. fruit extract as a possible mechanism.
Materials and Methods
Animals
Swiss mice of either sex, 6–8 weeks old, were used for all the experiments. The mice were bred and maintained in the animal house facility of Bhabha Atomic Research Centre. All the experimental protocols were as per the guidelines of the institutional animal ethics committee of Bhabha Atomic Research Centre, Government of India.
Extract preparation
Commercially available dried fruits of Tribulus terrestris. Linn. (Zygophyllaceae) were used in these studies. The specimen, authenticated on the basis of fruit and seed characteristics, has been deposited in the herbarium of the Department of Botany, Institute of Science, Mumbai (I. Sc. 1001). The dried fruit (500 g) was powdered and extracted with distilled water (1000 mL) for 24 h. The supernatant obtained after centrifugation at 800 × g for 10 min was lyophilized and is referred to as Tribulus terrestris. extract (TTE).
Cell preparation
Single cell suspension was prepared from the spleen by gentle teasing in RPMI 1640 Medium (containing 15 mM HEPES, 2 mM l-glutamine, 100 IU/mL penicillin, 100 µg/mL streptomycin, and 20 µM 2-mercaptoethanol; Sigma Chemical Company, St. Louis, MO, USA) with the help of a piston and sieve. Red blood cells were removed by lysis using 0.83% NH4Cl. The cells were washed three-times with the same medium, and the viability of the cell preparation was assessed by Trypan blue dye exclusion.
Detection of intracellular ROS
Intracellular ROS were estimated as described previously (Shankar et al., Citation2003). Briefly, cells were labeled with 20 µM dichlorodihydrofluorescein diacetate (H2DCFDA; Sigma) for 15 min at 37°C. Oxidative stress was induced by either exposure to γ.-radiation (dose rate; 6.64 Gy/min; total dose; 1 Gy) or by addition of 250 µM 2,2′-azobis.(2-propionimidinedihydrochloride) (AAPH). Radiation generates predominately hydroxyl radicals, and AAPH is a source of peroxyl radicals (Krasowska et al., Citation2000). The cells were acquired in a FACS Vantage flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA), and the increase in FL1 fluorescence intensity (530 nm) was calculated using CELLQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). Three replicates were taken for each group, and 20,000 cells were acquired per sample. The median fluorescence intensity of each sample was calculated using CELLQuest software. The results are expressed as the arithmetic mean of these values ± SE.
Assay for apoptosis
Spleen cells were exposed to a dose of 1 Gy in absence or presence of different concentrations of TTE and further cultured for 24 h. The cells were washed and labeled with hypotonic propidium iodide (PI) staining solution (50 µg/mL PI, 0.1% sodium citrate, and 0.1% Triton-X 100; Sigma) at 4°C overnight (Nicoletti et al., Citation1991). The cells were acquired (20,000 cells/sample) and analyzed by flow cytometry as described previously. The cells with < G0/G1 DNA content were enumerated as apoptotic cells, and the results are expressed as mean percent apoptotic cells for three replicates ± SEM.
Assay for proliferation
Spleen cells (1 × 106 cells/mL) were cultured with different concentrations of TTE for 48 and 72 h, respectively. The wells were pulsed with 1 µCi [3H]thymidine for 16 h and were harvested on a glass fiber paper. The incorporation of [3H]thymidine was measured in a liquid scintillation counter, and the results are expressed as mean counts per minute for six replicates ± SEM.
Statistical analyses
All the experiments were repeated two- to three-times. Student's t.-test was used to determine the significance of difference with respect to a given parameter between the groups, and p < 0.05 was considered significant.
Results
Effect of TTE on radiation- and AAPH-induced ROS
When the spleen cells were exposed to radiation in the presence of different concentrations of TTE, the median fluorescence intensity of dichlorofluorescein (DCF) was lower than that in the absence of TTE indicating lower levels of intracellular ROS. This reduction in ROS level by TTE was concentration-dependent, and maximum reduction (50%, p < 0.01) in ROS was observed at 100 µg/mL TTE (b). However, at 100 µg/mL, TTE showed pro-oxidant activity by increasing the basal ROS level as indicated by increased fluorescence intensity of the TTE-treated unirradiated cells (a). Similar concentration-dependent decrease in intracellular ROS was also observed in presence of TTE in AAPH-treated spleen cells. However, TTE was more effective in reducing AAPH-induced ROS [70% protection at 100 µg/mL (p < 0.01)] (c). As seen from and , there was only marginal reduction in radiation- or AAPH-induced ROS when the cells were washed after incubation with TTE for 1 h prior to γ.-irradiation or treatment with AAPH. Thus, presence of TTE is required at the time of induction of oxidative stress for optimal reduction in intracellular ROS.
Figure 1 Tribulus terrestris. extract inhibited both radiation- as well as AAPH-induced intracellular ROS. Spleen cells were labeled with H2DCFDA and exposed to either γ-radiation or AAPH in presence of different concentrations of TTE. The increase in intracellular fluorescence of cells was measured by flow cytometry after their treatment. (a) TTE only, (b) TTE immediately followed by exposure to a dose of 2 Gy γ radiation, (c) TTE immediately followed by exposure to 250 µM AAPH. The results are expressed as the average median fluorescence intensity ± SE for three replicates. Representative data from one of the three similar experiments are shown.
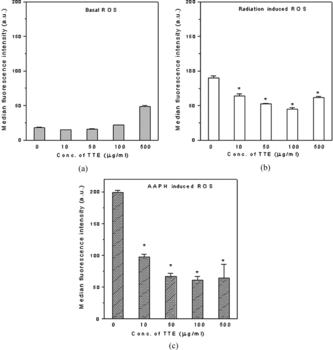
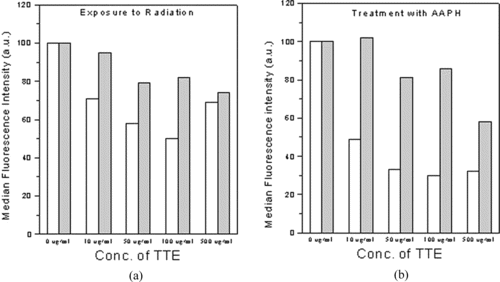
Figure 2 Presence of TTE during induction of oxidative stress was required for optimal protection against intracellular ROS. Cells treated with TTE for 1 h were subsequently exposed to (a) a dose of 2 Gy γ radiation, (b) 250 µM AAPH. All the samples were taken in triplicate, and the average median fluorescence intensity is expressed as the percent change over the control value. Open bars indicate ROS in cells exposed to 2 Gy in the presence of TTE. Closed bars indicate ROS in cells exposed to 2 Gy after removal of TTE. Data from one of the three similar experiments are shown.
Effect on radiation-induced apoptosis
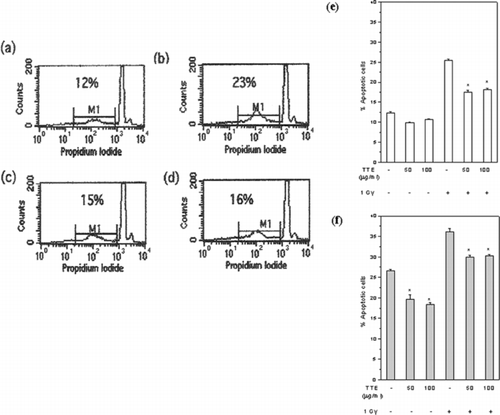
Exposure to radiation induced apoptosis in control cells as well as in cells treated with TTE (). However, there was a significant decrease (p < 0.01) in percent apoptotic cells treated with TTE and exposed to radiation immediately (–e). This protection was not further enhanced by preincubation of the cells with TTE for 24 h before irradiation (f). There was a significant decrease (p < 0.05) in the basal level of apoptosis in unirradiated cells when treated with different concentrations of TTE for 24 as well as 48 h ( and ).
Figure 3 TTE protected against radiation-induced apoptosis. Spleen cells were cultured for 24 h after irradiation and labeled with propidium iodide. Percent apoptotic cells were estimated as those with < G0/G1 DNA content. Flow cytometric profiles (a–d) and histograms (e, f) of different groups are shown: (a) control, (b) 1 Gy irradiated, (c) 1 Gy irradiated in presence of 50 µg/mL TTE, (d) 1 Gy irradiated in presence of 100 µg/mL TTE, (e) exposed to 1 Gy immediately after addition of 100 µg/mL TTE, or (f) exposed to 1 Gy 24 h after culture with 100 µg/mL TTE. All the samples were taken in triplicate, and the results are expressed as mean percent apoptotic cells ± SEM. Representative data from one of the three similar experiments are shown.
Effect on lymphocyte proliferation
There was an increase in [3H]thymidine incorporation in spleen cells cultured in presence of TTE with maximum incorporation observed at 100 µg/mL (). The response was higher at 48 h compared with that at 72 h. This suggested the presence of a mitogenic constituent in TTE.
Figure 4 TTE was mitogenic to murine spleen cells. Spleen cells were cultured with TTE, and the proliferation response was measured by [3H]-thymidine incorporation 48 and 72 h after culture. The samples were taken in six replicates, and the results are expressed as mean counts per minute ± SEM. Representative data from one of the three similar experiments are shown.
![Figure 4 TTE was mitogenic to murine spleen cells. Spleen cells were cultured with TTE, and the proliferation response was measured by [3H]-thymidine incorporation 48 and 72 h after culture. The samples were taken in six replicates, and the results are expressed as mean counts per minute ± SEM. Representative data from one of the three similar experiments are shown.](/cms/asset/d4eb20fc-1f70-43e8-a748-4003f35763e1/iphb_a_253748_f0004_b.gif)
Discussion
Under conditions of oxidative stress, cellular responses to ROS are critical in maintaining cellular functions and in making the decision between cell survival and death. The use of herbal remedies as modulators of oxidative stress in the treatment of many disorders is being actively explored. Qian-kun-nin, a Chinese herbal formulation, has been reported to attenuate oxidative stress and thus protect against cardiovascular diseases (Shao et al., Citation2001). Similarly, aqueous extracts of herbal plants like Hibiscus sabdariffa Linn.. (Malvaceae) Rosmarinus officinalis Linn.. (Lamiaceae), and Salvia officinalis Linn.. (Lamiaceae)have been shown to protect against oxidative stress-induced hepatotoxicity (Amin & Hamza, Citation2005).
The aqueous extract of T. terrestris. fruit is used in traditional medicine for the treatment of various disorders. The chemopreventive activity of aqueous extract of T. terrestris. root and fruit has been reported to be associated with an increase in reduced glutathione and a decrease in lipid peroxidation in treated mice (Kumar et al., Citation2006). In the current studies, we found direct reduction of ROS by TTE, which may be crucial for alleviation of oxidative stress. Incubation with this extract resulted in concentration-dependent reduction in the AAPH- or γ-radiation-induced intracellular ROS ( and ). Intracellular ROS consist of those generated within the cells and those that are generated extracellularly and permeate the cell membrane. The presence of the extract at the time of induction of oxidative stress was required for optimum reduction in ROS as removal of the extracellular TTE abrogated the ROS reducing effect () indicating that the extract was involved in the direct scavenging of the diffusible ROS after AAPH or radiation exposure. Preincubation of cells with TTE for 1 h did not significantly enhance the protection (data not shown). The presence of water-soluble polysaccharides in T. terrestris. extract has been reported (Huang et al., Citation1991). Polysaccharides, being very large molecules, cannot diffuse inside the cell. Therefore, it is possible that TTE may be scavenging extracellular diffusible ROS via the cell-impermeable constituents like polysaccharides. Protection against DNA damage by polysaccharides from T. terrestris. (Liu et al., Citation1995) and Tinospora cordifolia. (Menispermaceae) (Subramaniam et al., Citation2003) has been shown. Beyond 100 µg/mL, it also showed pro-oxidant activity as evident by the increase in basal ROS levels (a). A similar pro-oxidant effect has been reported for other well-known antioxidants such as ascorbic acid (Podmore et al., Citation1998) and β-carotene (Black, Citation2004).
Along with a significant reduction in intracellular ROS after irradiation or AAPH treatment, TTE also protected against radiation-induced apoptosis (). The mechanism of protection against ROS-induced damage in cells involves scavenging of ROS and induction of antioxidant defenses, which are temporally separated. The end result will depend on the relative contributions of the two mechanisms. For example, a well-known antioxidant N.-acetylcysteine, which gives 80–90% protection against radiation-induced ROS, did not protect against radiation-induced apoptosis when added just prior to irradiation (data not shown). However, when cells were incubated for 24 h with N.-acetylcysteine prior to irradiation, there was 30% reduction in radiation-induced apoptosis (Shankar et al., Citation2003). However, our results show that TTE protected against oxidative stress–induced cell death to the same extent when added immediately or 24 h prior to radiation exposure (f). Reduction in radiation-induced ROS by TTE when added simultaneously showed correlation with a decrease in radiation-induced cell death. TTE therefore does not appear to stimulate the intracellular antioxidant defenses. Thus, TTE primarily appears to be protecting the cells from radiation-induced death through scavenging of extracellular ROS, and does not involve induction of other antioxidant defense mechanisms.
TTE also showed mitogenic activity in spleen cells (). This may be due to presence of certain polysaccharide(s) and/or protein(s) in the extract. Mitogens are known to induce cellular ROS (Tatla et al., Citation1999) and play an important role in cellular activation (Williams & Kwon, Citation2004). The increase in intracellular ROS by TTE at ≥ 100 µg/mL could therefore be related to its mitogenic activity. It may be speculated that the mitogenic activity along with cytoprotective effect of TTE may help in the restoration of oxidative stress–induced damage. These may be of particular relevance for its anticancer activity (Kumar et al., Citation2006) wherein it may reduce oxidative damage and at the same time potentiates the immune system by inducing mitogenic activation. Further investigations are now needed to establish the exact mechanism of action and to identify the active bioingredient(s) of TTE in order to explain its therapeutic efficacy.
Acknowledgment
We thank Dr. T. Srinivasu, Department of Botany, Institute of Science (Mumbai, India) for his help in identification and authentication of the plant material.
References
- Al-Ali M, Wahbi S, Twaij H, Al-Badr A (2003): Tribulus terrestris.: Preliminary study of its diuretic and contractile effects and comparison with Zea mays.. J Ethnopharmacol 85: 257–260.
- Ali NA, Julich WD, Kusnick C, Lindequist U (2001): Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J Ethnopharmacol 74: 173–179.
- Amin A, Hamza AA (2005): Hepatoprotective effects of Hibiscus., Rosmarinus. and Salvia. on azathioprine-induced toxicity in rats. Life Sci 77: 266–278.
- Bedir A, Khan IA, Walker LA (2002): Biologically active steroidal glycosides from Tribulus terrestris.. Pharmazie 57: 491–493.
- Black HS (2004): Pro-carcinogenic activity of beta-carotene, a putative systemic photoprotectant. Photochem Photobiol Sci 3: 753–758.
- Brown GA, Vukovich MD, Martini ER, Kohut ML, Frank WD, Jackson DA (2001): Endocrine and lipid responses to chronic androstenendiol–herbal supplementation in 30–58 year old men. J Am Coll Nutr 20: 520–528.
- Buttke TM, Sandstrom PA (1994): Oxidative stress as a mediator of apoptosis. Immunol Today 15: 7–10.
- Deepak M, Dipankar G, Prashanth D, Asha MK, Amit A, Venkataraman BV (2002): Tribulosin and β-sitosterol-d-glucoside, the antihelminthic principles of Tribulus terrestris.. Phytomed 9: 753–756.
- Gauthaman K, Adaikan PG (2005): Effect of Tribulus terrestris. on nicotinamide adenine dinucleotide phosphate-diaphorase activity and androgen receptors in rat brain. J Ethnopharmacol 96: 127–132.
- Halliwell B, Gutteridge JMC (1989): Oxidative stress: adaptation, damage, repair and death in Free Radicals in Biology and Medicine, 3rd Ed. London, Oxford University Press, pp. 1–36.
- Huang XL, Zhang YS, Liang ZY (1991): Studies on water soluble polysaccharides isolated from Tribulus terrestris. L. purification and preliminary structural determination of heteropolysaccharide H. Acta Pharmaceutica Sinica 26: 578–583.
- Ichikawa H, Konishi T (2002): In vitro. antioxidant potentials of traditional Chinese medicine, Shengmai San and their relation to in vivo. protective effect on cerebral oxidative damage in rats. Biol Pharma Bull 25: 898–903.
- Ichiki T, Tokunou T, Fukuyama K, Lino N, Masuda S, Takeshita A (2003): Cyclic AMP response element-binding protein mediates reactive oxygen species-induced c-fos expression. Hypertension 42: 177–183.
- Kim HY, Yokozawa T, Cho EJ, Yamabe N (2004): Protective effects of the Chinese prescription Hachimi-jio-gan against diabetic oxidative stress. J Pharm Pharmacol 56: 1299–1305.
- Krasowska A, Lukaszewicz M, Oswiecimska M, Witek S, Sigler K (2000): Spontaneous and radical-induced plasma membrane lipid peroxidation in differently oxidant-sensitive yeast species and its suppression by antioxidants. Folia Microbiol (Praha) 45: 509–514.
- Kumar M, Soni AK, Shukla S, Kumar A (2006): Chemopreventive potential of Tribulus terrestris. against 7,12-dimethylbenz(a.)anthracene induced skin papillomagenesis in mice. Asian Pac J Cancer Prev 7: 289–294.
- Li JX, Shi Q, Xiong QB, Prasain JK, Tezuka Y, Hareyama T, Wang ZT, Tanaka K, Namba T, Kadota S (1998): Tribulusamide A and B, new hepatoprotective lignanamides from the fruits of Tribulus terrestris.: Indications of cytoprotective activity in murine hepatocyte culture. Planta Med 64: 628–631.
- Liu Q, Chen Y, Wang J, Chen X, Han Y (1995): A study of the protective effects of Tribulus terrestris. L., polysaccharide on genetic damage. Zhongguo Zhong Yao Za Zhi 20: 427–429.
- Ljubuncic P, Azaizeh H, Portnaya I, Cogan U, Said O, Saleh KA, Bomzon A (2005): Antioxidant activity and cytotoxicity of eight plants used in traditional Arab medicine in Israel. J Ethnopharmacol 99: 43–47.
- Margaill I, Plotkine M, Lerouet D (2005): Antioxidant strategies in the treatment of stroke. Free Radic Biol Med 39: 429–443.
- Matsuzawa A, Ichijo H (2005): Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid Redox Signal 7: 472–481.
- Nakashima I, Takeda K, Kawamoto Y, Okuno Y, Kato M, Suzuki H (2005): Redox control of catalytic activities of membrane-associated protein tyrosine kinases. Arch Biochem Biophys 434: 3–10.
- Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C (1991): A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 139: 271–279.
- Ozben T (1998): Mechanisms involved in neuronal damage. In: Free radicals, oxidative stress and antioxidants, NATO ASI series. New York, Plenum Press, pp. 163–189.
- Phillips OA, Mathew KT, Oriowo MA (2006): Antihypertensive and vasodilator effects of methanolic and aqueous extracts of Tribulus terrestris. in rats. J Ethnopharmacol 104: 351–355.
- Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J (1998): Vitamin C exhibits pro-oxidant properties. Nature 392: 559.
- Rice-Evans CA, Diplock AT (1992): Current status of antioxidant therapy. Free Radic Biol Med 15: 77–96.
- Shankar B, Kumar SS, Sainis KB (2003): Generation of reactive oxygen species and radiation response in lymphocytes and tumor cells. Radiat Res 160: 478–487.
- Shao Z, Li C, Becker LB, Vanden Hoek TL, Schumacker PT, Attele AS, Zhang L, Xie J, Yuan C (2001): Qian-Kun-Nin, a Chinese herbal medicine formulation, attenuates mitochondrial oxidant stress in cardiomyocytes. J Ethnopharmacol 74: 63–68.
- Sharifi AM, Darabi R, Akbarloo N (2003): Study of antihypertensive mechanism of Tribulus terrestris. in 2K1C hypertensive rats: Role of tissue ACE activity. Life Sci 73: 2963–2971.
- Subramaniam M, Chintalwar GJ, Chattopadhyay S (2003): Radioprotective property of poysachharide in Tinospora cordifolia.. Ind J Biochem Biophys 40: 22–26.
- Tatla S, Woodhead V, Foreman JC, Chain BM (1999): The role of reactive oxygen species in triggering proliferation and IL-2 secretion in T cells. Free Radic Biol Med 26: 14–24.
- Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J (2004): Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 266: 37–56.
- Williams MS, Kwon J (2004): T cell receptor stimulation, reactive oxygen species, and cell signaling. Free Radic Biol Med 37: 1144–1151.
- Zhang JD, Xu Z, Cao YB, Chen HS, Yan L, An MM, Gao PH, Wang Y, Jia XM, Jiang YY (2006): Antifungal activities and action mechanisms of compounds from Tribulus terrestris. L. J Ethnopharmacol 103: 76–84.