Abstract
Free radicals generated from oxidative stress (OS) have been depicted in the causation of cancerous and noncancerous diseases in humans. Increase in fat content of the body may favor the deleterious effect of free radical attack. The generation of free radicals is enhanced in respiratory burst during bacterial infection. The level of plasma membrane cholesterol appears to be critical in the regulation of microbial entry, intracellular trafficking, and exit. The current study was designed to compare the in vitro. antibacterial and antioxidant activities of hypocholesterolemic drugs atorvastatin and simvastatin. Agar-well diffusion assay was used to screen the antibacterial activity using Gram-positive and Gram-negative bacterial strains. Antioxidant activity was evaluated using Fe2+-induced lipid peroxidation inhibiting activity in whole rat liver homogenate and Fe3+ reducing activity using ferric-reducing antioxidant power (FRAP) assay. Atorvastatin and simvastatin inhibited the growth of all bacterial strains tested. The zone of inhibition produced by atorvastatin is higher than that of simvastatin. However, antioxidant activities of simvastatin were higher than those of atorvastatin. The exhibited pleiotropic activities of these statins suggest their clinical advantages against bacterial infection and oxidative stress–induced human ailments apart from their wide use for hypolipidemic effects.
Introduction
Extensive research over the past decades indicates the etiologic role of free radicals in cancerous and noncancerous diseases in humans (Wiseman & Halliwell, Citation1996; West, Citation2000). Biologically relevant free radicals, which include reactive oxygen species (ROS) and reactive nitrogen species (RNS), are produced from activated phagocytes such as monocytes, neutrophils, eosinophils, and macrophages at the site of inflammation (Babior, Citation1999). This generation of ROS has been reported to be due to the activation of Nicotinamide adenine dinucleotide phosphate reduced (NADPH)>) oxidase enzyme during phagocytosis (Babior et al., Citation2002). A direct link between NADPH oxidase and lipopolysaccharide (LPS) induced activation of innate immunity and inflammation has been proposed recently (Janssen-Heininger et al., 2000). LPS, an integral component of the outer membrane of Gram-negative bacteria, can activate NADPH oxidase (Park et al., Citation2004). Cholesterol confined in lipid rafts is a crucial component required by microorganisms to enter the intracellular compartment (Goluszko & Nowicki, Citation2005). The generated free radicals from the inflammatory process can oxidize the low-density lipoprotein (LDL) cholesterol to increase the process of atherogenesis and can also participate in the pathophysiology of many diseases including cancer. Hence, it is worthwhile to evaluate antibacterial and antioxidant activities using lipid-lowering drugs—statins.
3-Hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase inhibitors (statins) are widely used drugs in the treatment of hypercholesterolemia, thereby reducing the risk of myocardial infarction (MI) and stroke (Blauw et al., Citation1998; Endres et al., Citation1998; Farnier & Davignon, Citation1998). They might also be useful in the therapy of other disorders, including Alzheimer disease (Crisby et al., Citation2002), osteoporosis (Waldman & Kritharides, Citation2003), and, in particular, for the treatment of cancer (Chan et al., Citation2003). Among the statins evaluated for their anticarcinogenic activities, lovastatin has been thoroughly studied (Jakobisiak & Golab, Citation2003). The emerging interests in their use as anticancer agents are based on preclinical evidence of their antiproliferative, proapoptotic, anti-invasive, and radiosensitizing properties (Chan et al., Citation2003; Ajith et al., Citation2006). Antimutagenicity of statins has also been recently reported (Ajith & Soja, Citation2006). However, an in vitro. comparative study on the direct effect of atorvastatin and simvastatin on bacterial growth or their antioxidant activities has not yet been reported. Free radicals generated from inflammatory sites can induce lipid peroxidation, and excess body fat will enhance the deleterious effects of free radicals. Therefore, the current study was designed to compare the in vitro. antioxidant activity and determination of antibacterial potential of hypolipaedemic drugs atorvastatin and simvastatin
Materials and Methods
Chemicals
Nutrient agar was purchased from Hi-media (Mumbai, India). Dimethyl sulfoxide (DMSO) was purchased from Merck India Ltd. (Mumbai, India). Ferrous ammonium sulfate [FeSO4(NH4)2SO4·6H2O], ascorbic acid, butanol, pyridine, and sodium dodecyl sulfate (SDS) were purchased from Qualigens Fine Chemicals (Mumbai, India). 2,4,6-Tripyridyl-S.-triazine (TPTZ) was from LOBA Fine Chemicals Pvt Ltd. (Mumbai, India). Atorvastatin (Aztor; Sun Pharmaceuticals Industries, Thrissur, Kerala, India), simvastatin (Simvas; Microlab, Pondicherry, India), and gentamicin (Nicholas, Mumbai, India) were purchased from the Amala Hospital Pharmacy. All other reagents used were of analytical grade.
Bacterial strains
Gram-positive organisms Staphylococcus aureus. Rosen (MTCC 96), Bacillus subtilis. (Ehreu) Cohn (MTCC 411) and Gram-negative organisms Escherichia coli. (Migu.) Caste & Chalm (MTCC 443) and Salmonella typhimurium. (Loeff.) Caste & Chalm (MTCC 98) were obtained from the Microbial Type Culture Collection, Institute of Microbial Technology, Chandigarh, India. All the strains were maintained on nutrient agar medium.
Determination of antibacterial activity
Antibacterial activity was determined using the agar-well diffusion method with Gram-positive and Gram-negative bacterial strains (Sheena et al., Citation2004). Briefly, bacterial inoculum (optical density 0.250 ± 0.010 at 600 nm in nutrient broth) was prepared from freshly grown overnight cultures. The inoculum was mixed with 20 mL of molten nutrient agar (2% v/v) at 45°C. Different concentrations of the statins (100 µg to 2 mg/well; dissolved in DMSO) were added into the wells (8-mm diameter) of the nutrient agar plates prepared using a sterile cork-borer. Plates were incubated for 48 h at 37°C, and the diameter of the inhibition zone was measured in two perpendicular directions. Plates were kept for 1 week at room temperature to observe the overgrowth of bacteria in the inhibition zone. Gentamicin (40 µg/well) was used as the standard reference drug.
Determination of in vitro. antioxidant activity
In vitro lipid peroxidation inhibiting activity.
In vitro. lipid peroxidation was determined according to the method of Ohkawa et al. (Citation1979). The reaction mixture contained rat whole liver homogenate 0.1 mL (25%, w/v) in Tris HCl buffer (20 mM, pH 7.0), KCl (150 mM), FeSO4 (NH4)2SO4·6H2O (0.16 mM), ascorbate (0.06 mM), and various concentrations of atorvastatin or simvastatin (0.1–2 mg in DMSO) in a final volume of 0.5 mL. The reaction mixture was incubated at 37°C for 1 h. After the incubation period, 0.2 mL SDS (8.1%), 1.5 mL TBA (0.8%), and 1.5 mL acetic acid (20% v/v, pH 3.5) was added. The total volume was made up to 4 mL with distilled water and then kept in water bath at 95–100°C for 1 h. After cooling, 1 mL of distilled water and 5 mL of n.-butanol and pyridine mixture (15:1) were added to the reaction mixture. The butanol:pyridine layer was removed by centrifuging at 2750 × g. for 10 min, and the absorbance at 532 nm was measured against the reagent blank in a spectrophotometer (ELICO, SL 164, UV-VS double beam spectrophotometer). Inhibition of lipid peroxidation was determined by comparing the absorbance of treated with that of control. Vitamin E was used as the reference standard.
Determination of ferric ion reducing activity
Fe3+ ion reducing activity of statins was determined by ferric-reducing antioxidant power (FRAP) assay (Lakshmi et al., Citation2004). Various concentrations of atorvastatin or simvastatin (0.05–0.5 mg/mL in DMSO) were incubated with FeCl3 in presence of TPTZ (10 mM) for 30 min at 37°C. The reducing effect of statins was measured as an increase in absorbance at 595 nm against a reagent blank. Ascorbic acid was used as the reference standard.
All experiments were repeated twice in triplicate for each concentration.
Results and Discussion
Atorvastatin and simvastatin () showed marked activity against all the bacterial strains tested (). Maximum zone of inhibition was produced by statins at 1 and 2 mg/well (). However, atorvastatin at lower concentration did not produce an inhibition zone. The inhibitory effect of atorvastatin was higher than that of simvastatin. No growth of bacteria was observed in the zone of inhibition even after 1 week at room temperature. All the bacterial strains were sensitive to gentamicin at a concentration of 40 µg/well. The exact mechanism of antibacterial activity exhibited by the statins is unknown. The direct cytotoxic effect of statins may possibly relate to the antibacterial activity. Phenolic compounds and aromatic alcohols are reported to have antibacterial activity mediated by growth inhibition, lethal effect, and cytologic damage (Lucchini et al., Citation1990). The presence of phenolic hydroxyl groups in simvastatin and more hydroxyl groups in atorvastatin may correlate with their antibacterial activity.
Table 1.. Antibacterial activity of atorvastatin and simvastatin
Figure 2 Antibacterial activity of atorvastatin [(a) 1 and (b) 2 mg/well)] against (A) Salmonella typhimurium. (MTCC 98); (B) Escherichia coli. (MTCC 443), and (C) Bacillus subtilis. (MTCC 411). (D) Effect of simvastatin against Escherichia coli. (MTCC 443) [(c) 1 and (d) 2 mg/well]. The reference drug, gentamicin (40 µg/well), was placed in the center well.
![Figure 2 Antibacterial activity of atorvastatin [(a) 1 and (b) 2 mg/well)] against (A) Salmonella typhimurium. (MTCC 98); (B) Escherichia coli. (MTCC 443), and (C) Bacillus subtilis. (MTCC 411). (D) Effect of simvastatin against Escherichia coli. (MTCC 443) [(c) 1 and (d) 2 mg/well]. The reference drug, gentamicin (40 µg/well), was placed in the center well.](/cms/asset/d7e0d474-1baa-4611-b130-dc2823e3e2c2/iphb_a_257354_f0002_b.gif)
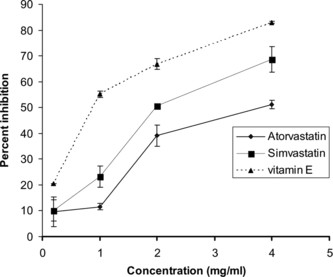
Atorvastatin and simvastatin significantly and dose-dependently inhibited the lipid peroxidation induced by Fe2+-ascorbate system in rat liver homogenate (). The generation of thiobarbituric acid reacting substances (TBARS) was inhibited by simvastatin at 4 mg/mL (68.6 ± 4.9%). However, the inhibition produced by atorvastatin at 4 mg/mL was found to be 51.2 ± 1.6%. The concentration to inhibit 50% of Fe2+-induced lipid peroxidation (IC50) was found to be 4.2 ± 0.2 and 2.1 ± 0.5 mg/mL for atorvastatin and simvastatin, respectively. The standard reference drug, vitamin E, showed an IC50 of 0.80 ± 0.05 mg/mL.
Figure 3 Lipid peroxidation inhibiting effects of atorvastatin, simvastatin, and vitamin E. Values are mean ± SD, n = 3.
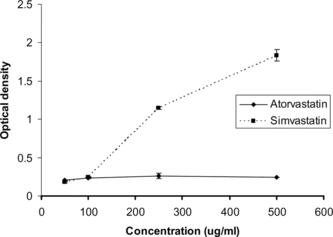
The metal-reducing effect of atorvastatin and simvastatin is given in . Simvastatin showed a dose-dependent reduction of Fe3+ ion. However, atorvastatin did not show a dose-dependent response. Most of the nonenzymatic antioxidants act as a reductant by their hydrogen-donating capacity. The reduction of Fe3+ to Fe2+ by statin favored the Fe2+-TPTZ complex formation. Nevertheless, the reducing property was less than that of the standard antioxidant, ascorbic acid. Ascorbic acid at 1 µg/mL showed high reducing effect as evident from the optical density of 0.950 ± 0.08. The exhibited lipid peroxidation inhibiting activity of these statins may be partially related to their reducing property. The hydroperoxyl radical formed during the lipid peroxidation chain reaction pathway might be reduced to a stable lipid hydroperoxide by the statins. The higher antioxidant activity of simvastatin when compared with atorvastatin may be related to its phenolic hydroxyl group. Nakamura et al. (Citation2000) reported the mechanism of the antioxidant activity of fluvastatin and its metabolites due to the presence of the phenolic hydroxyl group.
Figure 4 Ferric ion reducing effect of atorvastatin and simvastatin in FRAP assay. Values are mean ± SD, n = 3.
Antioxidants represent an important defense against cellular injury mediated by the free radicals. Wassmann et al. (Citation2002) demonstrated the cellular antioxidant effects of atorvastatin in cultured vascular smooth muscle cells of rat by decreasing the NADPH-oxidase expression and upregulating the expression of catalase. Simvastatin can also promote the intracellular antioxidant system by restoring the endothelial superoxide dismutase (SOD) function (Carneado et al., Citation2002). Moreover, increase and no increase in serum paraoxonase (PON1) activity, a high-density lipoprotein (HDL)-bound antioxidant enzyme, have been reported respectively in simvastatin and atorvastatin treated patients (Tomas et al., Citation2000; Sardo et al., Citation2005). Recently, statins were found to decrease intracellular bacterial proliferation (Catron et al., Citation2004). The direct antibacterial activity demonstrated in this study further supports their efficacy in preventing bacterial infection.
The results of this in vitro. study concluded that atorvastatin and simvastatin are effective antioxidant and antibacterial agents. The antibacterial activity is higher for atorvastatin, and antioxidant activity is higher for simvastatin. Nevertheless, their comparative activities exhibited the pleiotropic properties of statins and suggest their clinical advantages against bacterial infection and oxidative stress–induced human ailments apart from their wide use for hypolipidemic effects.
Acknowledgments
The valuable help of Dr. K.K. Janardhanan, Professor, Department of Microbiology, Amala Cancer Research Centre (Amala Nagar, Thrissur, Kerala, India) for the antibacterial activity work and the help of Babitha Ajith, Department of English, St'Alosius HSS (Elthuruthu, Thrissur, Kerala, India) during the preparation of this manuscript are gratefully acknowledged.
References
- Ajith TA, Harikumar KB, Thasna H, Sabu MC, Babitha NV (2006): Proapoptotic and antitumor activities of the HMG-CoA reductase inhibitor, lovastatin, against Dalton's lymphoma ascites tumor in mice. Clin Chim Acta 366: 322–328.
- Ajith TA, Soja M (2006): A comparative study on the antimutagenicity of atorvastatin and lovastatin against directly acting mutagens. Cell Biol Toxicol 22: 269–274.
- Babior BM (1999): NADPH oxidase: An update. Blood 93: 1464–1476.
- Babior BM, Lambeth JD, Nauseef W (2002): The neutrophil NADPH oxidase. Arch Biochem Biophys 397: 342–344.
- Blauw GJ, Lagaay AM, Westendorp RG (1998): Statins for prevention of stroke. Lancet 352: 144.
- Carneado J, Jimenez L, Herrera MD, Pamies E, Martin-Sanz MD, Stiefel P, Miranda M, Bravo L, Marhuenda E (2002): Simvastatin improves endothelial function in spontaneously hypertensive rats through a superoxide dismutase mediated antioxidant effect. J Hypertens 20: 429–437.
- Catron DM, Lange Y, Borensztajn J, Sylvester MD, Jones BD, Haldar K (2004): Salmonella enterica. serovar typhimurium. requires non-sterol precursors of the cholesterol biosynthetic pathway for intracellular proliferation. Infect Immun 72: 1036–1042.
- Chan KKW, Oza AM, Siu LL (2003): The statins as anticancer agents. Clin Cancer Res 9: 10–19.
- Crisby M, Carlson LA, Winblad B (2002): Statins in the prevention and treatment of Alzheimer disease. Alzheimer Dis Assoc Disord 16: 131–136.
- Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK (1998): Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci USA 95: 8880–8885.
- Farnier M, Davignon J (1998): Current and future treatment of hyperlipidemia: The role of statins. Am J Cardiol 82: 3J–10J.
- Goluszko P, Nowicki B (2005): Membrane cholesterol: A crucial molecule affecting interactions of microbial pathogens with mammalian cells. Infect. Immun 73: 7791–7796.
- Jakobisiak M, Golab J (2003): Potential antitumor effects of statins (review). Int J Oncol 23: 1055–1069.
- Lakshmi B, Tilak JC, Adhikari S, Devasagayam TPA, Janardhanan KK (2004): Evaluation of antioxidant activity of selected Indian mushrooms. Pharm Biol 42: 179–185.
- Lucchini JJ, Corre J, Cremieux A (1990): Antibacterial activity of phenolic compounds and aromatic alcohols. Res Microbiol 141: 499–510.
- Nakamura T, Nishi H, Kokusenya Y, Hirota K, Miura Y (2000): Mechanism of antioxidative activity of fluvastatin-determination of the active position. Chem Pharm Bull (Tokyo) 48: 235–237.
- Ohkawa H, Ohishi N, Yagi K (1979): Assay for lipid peroxide in animal tissues by thiobarbituric acid reaction. Ann Biochem 95: 351–358.
- Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS (2004): Cutting edge: Direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappaB. J Immunol 173: 3589–3593.
- Sardo MA, Campo S, Bonaiuto M, Bonaiuto A, Saitta C, Trimarchi G, Castaldo M, Bitto A, Cinquegrani M (2005): Antioxidant effect of atorvastatin is independent of PON1 gene T(-107)C, Q192R and L55M polymorphisms in hypercholesterolaemic patients. Curr Med Res Opin 21: 777–784.
- Sheena N, Ajith TA, Mathew T, Janardhanan KK (2004): Antibacterial activity of three macrofungi, Ganoderma lucidum, Navesporus floccosa. and Phellinus rimosus. occurring in South India. Pharm Biol 41: 564–567.
- Tomas M, Senti M, Garcia-Faria F, Vila J, Torrents A, Covas M, Marrugat J (2000): Effect of simvastatin therapy on paraoxonase activity and related lipoproteins in familial hypercholesterolemic patients. Arterioscler Thromb Vasc Biol 20: 2113–2119.
- Waldman A, Kritharides L (2003): The pleiotropic effects of HMG-CoA reductase inhibitors: Their role in osteoporosis and dementia. Drugs 63: 139–152.
- Wassmann S, Laufs U, Muller K, Konkol C, Ahlbory K, Baumer AT, Linz W, Bohm M, Nickeing G (2002): Cellular antioxidant effects of atorvastatin in vitro. and in vivo.. Arterioscler Thromb Vasc Biol 22: 300–305.
- West IC (2000): Radicals and oxidative stress in diabetes. Diabet Med 17: 171–180.
- Wiseman H, Halliwell B (1996): Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem J 313: 17–29.