Abstract
This study investigates the putative hepatoprotective and antioxidant activity of silymarin (SIL) on diethylnitrosamine (DEN)-induced liver damage in male Wistar rats. A single intraperitoneal administration of DEN (200 mg/kg) to rats resulted in significantly elevated serum levels of AST, ALT, ALP, LDH, γ-GT, and BIL compared with controls after 30 days in serum. In contrast, the liver tissue showed a significant decrease in the levels of AST, ALT, and ALP, and an increase in LDH and γ-GT. Elevated levels of lipid peroxidation (LPO) were observed in the liver tissue after DEN administration. Quantitative analysis of catalase (CAT) and superoxide dismutase (SOD) revealed lower activities of these key antioxidant enzymes in the liver upon DEN administration. The status of antioxidant vitamins, vitamin C and vitamin E, were also found to be decreased in DEN-exposed rats. When rats with DEN-induced hepatotoxicity were treated with SIL (50 mg/kg, orally) for 30 days, the levels of AST, ALT, ALP, LDH, γ-GT, and BIL reverted to near normalcy, whereas the hepatic concentration of CAT, SOD, vitamin C, and vitamin E were significantly increased, and that of LPO significantly lowered, when compared with DEN-exposed, untreated rats. These results suggest that SIL is able to significantly alleviate the hepatotoxicity and oxidative stress induced by DEN in rats.
Introduction
Silymarin (SIL), a flavanolignan, is extracted from the seeds and fruits of the plant Silybum marianum. Gaertn. (Compositae). The main flavanolignans in SIL are silibin (also known as silibinin), silydianin, isosilybin, and silychristin. Silibin, which constitutes 50% to 70% of the flavonoids, is regarded as the single most important component of SIL. Extracts obtained from Silybum marianum. (also called “milk thistle”) have been used since the fourth century BC for the treatment of plague and congestive conditions of the liver and spleen (Choksi et al., Citation2000). Seeds of Silybum marianum. have been used for more than 2000 years to treat liver and gallbladder disorders including hepatitis, cirrhosis and jaundice, and to protect the liver against poisoning from chemical and environmental toxins, including snakebites, insect stings, mushroom poisoning, and alcohol (Kren & Walterova, Citation2005). In European countries, SIL is widely used for protection against various hepatobiliary problems such as hepatitis, cirrhosis, gallstones, and jaundice (Flora et al., Citation1998). Due to its vast proven pharmacological effects, the German Commission E has recommended it for the treatment of dyspeptic complaints, toxin-induced liver damage, and liver cirrhosis, and as supportive therapy for chronic liver inflammatory conditions (Belmenthal, Citation1998). It is also reported to offer protection against chemical hepatotoxins such as CCl4 (Muriel & Mourelle, Citation1990), acetaminophen (Muriel et al., Citation1992), alcoholic liver diseases (Feher et al., Citation1989), phalloidin, galactosamine, and thioacetamide (Fraschini et al., Citation2002). Due to its hepatoprotective and antioxidant properties, SIL has been recommended for use as an adjunct in the treatment of liver diseases, although its clinical efficacy is continuously discussed (Flora et al., Citation1998; Saller et al., Citation2001). In light of these findings, we decided to test its efficacy against diethylnitrosamine (DEN)-induced hepatocellular damage as studies in this regard were meager.
DEN is a N.-nitroso alkyl compound, categorized as a potent hepatotoxin and hepatocarcinogen in experimental animals, producing reproducible tumors after repeated administration (Jose et al., Citation1998). DEN is also used as a hepatotoxic agent to induce hepatocellular necrosis in experimental animals (Pitot et al., Citation1989). The main reason for concern is that DEN is widely found in a variety of foods like cheese, soybean, smoked, salted, and dried fish, cured meat, alcoholic beverages, and groundwater having high level of nitrates (Liao et al., Citation2001). Metabolism of certain therapeutic drugs is also reported to produce DEN (Akintonwa, Citation1985). DEN is also detected in tobacco smoke at a concentration of 1 to 28 ng/cigarette and in baby bottle nipples at a level of 10 ppb (IARC, Citation1972). Considering the above facts, it is clear that humans are at potential risk for exposure to DEN. As human exposure to DEN has become inevitable, development of an effective hepatoprotective agent has become the need of the day. Various plants and plant-derived products have found to be effective against DEN-induced hepatocarcinogenesis and hepatotoxicity (Rajeshkumar Ahmed et al., Citation2001; Yoshikawa et al., Citation2001; Barbisan et al., Citation2003).
As oxidative stress plays a central role in liver disease pathogenesis and progression, antioxidants have been proposed as therapeutic agents, as well as drug co-adjuvant, to counteract liver damage (Vitaglione et al., Citation2004). Although the protective antioxidant effect of SIL has been well documented against a wide range of hepatotoxins, its efficacy against DEN-induced hepatotoxicity has not been studied earlier. Hence, we decided to investigate the hepatoprotective and antioxidant properties of SIL against DEN-induced hepatotoxicity using rats as experimental model.
Materials and Methods
Animals
Wistar albino male rats weighing 200 ± 10 g, purchased from Tamil Nadu Veterinary and Animal Sciences University, Chennai, were used in the study. The animals were randomly divided into four groups with six animals in each group and were housed in polypropylene cages in the departmental animal house. Animals were maintained at 25 ± 2°C with 12-h light and dark cycle. They were fed standard pellet feed and water ad libitum.. Animals were allowed to acclimatize to the animal house conditions before the study. Prior approval to perform animal experimentation was obtained from the institutional animal ethics committee (IAEC), and all animal experiments were performed in accordance with the strict guidelines prescribed by the IAEC.
Chemicals
DEN and 1,1,3,3-tetraethoxypropane were purchased from Sigma Chemical Company (St. Louis, MO, USA). SIL was obtained as a gift from Central Drug Research Institute (CDRI), Lucknow, India. All other chemicals used were of analytical grade, purchased locally, and were used without further purification.
Experimental design
Rats were divided into four groups with six animals in each group. The experimental design was as follows:
Group I: This group served as control and was treated with saline orally for 30 days.
Group II: Rats were administered a single dose of DEN (200 mg/kg b.w., i.p.) on day 0 and left for 30 days without any treatment.
Group III: Rats were administered DEN (200 mg/kg b.w., i.p.) on day 0 followed by SIL (50 mg/kg b.w., p.o.) from day 1 until day 30.
Group IV: Rats were treated with SIL (50 mg/kg b.w., p.o.) alone from day 1 until day 30.
At the end of the experimental period, animals were subjected to ether anesthesia, blood was collected from retroorbital plexus, and serum was separated by centrifugation. Animals were sacrificed by cervical decapitation, and the liver was excised, washed in ice-cold saline, and blotted to dryness. A 1% homogenate of the liver tissue was prepared in Tris-HCl buffer (0.1 M; pH 7.4), centrifuged, and the clear supernatant used for further biochemical assays. All the biochemical estimations were completed within 24 h of animal sacrifice.
Biochemical analysis
Aspartate and alanine transaminases (AST and ALT) were assayed in the serum and liver tissue homogenate according to the method of Wooten (Citation1964). Alkaline phosphatase (ALP) was assayed in the serum and liver tissue homogenate according to King (Citation1965a). Lactate dehydrogenase (LDH) was assayed by the method of King (Citation1965b). The method is based on the ability of LDH to form pyruvate in the presence of coenzyme NAD+. The pyruvate formed was made to react with 2,4-dinitrophenylhydrazine in hydrochloric acid. The hydrazone formed turns into an orange-colored complex in alkaline medium, which was measured at 420 nm. γ-Glutamyl transferase (γ-GT) in serum and liver was estimated as described by Rosalki and Rau (Citation1972). Protein content was estimated by the method of Lowry et al. (Citation1951). Total serum bilirubin (BIL) was estimated by the method of Malloy and Evelyn (Citation1937).
Lipid peroxidation (LPO) was determined in the liver tissue as described by Ohkawa et al. (Citation1979). Malondialdehyde (MDA), formed as an end product of the peroxidation of lipids, served as an index of the intensity of oxidative stress. MDA reacts with thiobarbituric acid to generate a colored product that has absorption maxima at 532 nm.
Superoxide dismutase (SOD) was assayed according to the method of Marklund and Marklund (Citation1974). The degree of inhibition of autooxidation of pyrogallol at an alkaline pH by SOD was used as a measure of the enzyme activity. Catalase (CAT) was assayed by the method of Sinha (Citation1972). In this method, dichromate in acetic acid was reduced to chromic acetate when heated in the presence of hydrogen peroxide (H2O2), with the formation of perchloric acid as an unstable intermediate. Chromic acetate thus produced was measured colorimetrically at 570 nm. Vitamin C (Vit C) was assayed by the procedure of Omaye et al. (Citation1979). Vitamin C was oxidized by copper to form dehydroascorbic acid and diketoglutaric acid, which were treated with 2,4-dinitrophenylhydrazine to form the derivative of bis-2,4-dinitrophenylhydrazine. This compound in strong sulfuric acid undergoes rearrangements to form a product that is measured at 520 nm. Vitamin E (Vit E) in the liver tissue was estimated as described by Varley et al. (Citation1976). This method is based on the reduction of ferric ions to ferrous ions by the tocopherols in the presence of α,α′-dipyridyl, resulting in the formation of red color, which was measured spectrophotometrically.
Statistical analysis
The results were expressed as mean ± standard error (SE) for six animals in each group. Differences between groups were assessed by one-way analysis of variance (ANOVA) using the SPSS software package for Windows (version 7.5). Post hoc testing was performed for intergroup comparisons using Tukey's multiple comparison; significance at p values < 0.001, < 0.01, < 0.05 have been given respective symbols.
Results
Effect of SIL treatment on hepatic markers in serum
presents the activities of AST, ALT, and ALP in the serum of control and experimental animals. Administration of a single dose of DEN (group II) produced a significant increase in the activities of all the three marker enzymes, the increase being 2-fold for AST and ALP and 3-fold for ALT. Treatment with SIL after DEN administration (group III) significantly decreased the activities of these enzymes, whereas no significant alteration was observed in rats treated with SIL alone (group IV). depicts the activities of γ-GT, LDH, and BIL in the serum of control and experimental animals. There was a similar increase in the activities of these enzymes also with administration of DEN (group II). Treatment with SIL after DEN administration markedly decreased the activities of these enzymes in group III rats.
Table 1.. Effect of DEN and SIL on the status of AST, ALT, and ALP in serum of experimental animals.
Table 2.. Effect of DEN and SIL on the status of γ-GT, LDH, and BIL in serum of experimental animals.
Effect of SIL treatment on hepatic markers in liver tissue
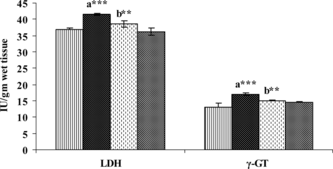
In contrast with the observations in serum, the liver tissue produced a highly significant fall in the levels of AST, ALT, and ALP (), whereas it produced a significant increase in the levels of LDH and γ-GT () upon administration of DEN. Treatment with SIL for 30 days after DEN administration significantly reduced the altered levels of these enzymes.
Figure 1 Figure 1 Effect of DEN and SIL on the status of AST, ALT, and ALP in liver tissue of experimental animals. Results are given as mean ± SE for six rats. Comparisons are made between: a, control rats (group I); b, DEN-treated rats (group II). The symbol (***) represents statistical significance at p<0.001. ![]()
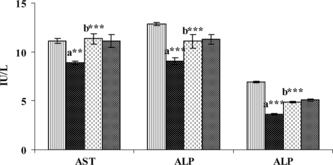
Figure 2 Figure 2 Effect of DEN and SIL on the status of LDH γ-GT in liver tissue of experimental animals. Results are given as mean ± SE for six rats. Comparisons are made between: a, control rats (group I); b, DEN-treated rats (group II). The symbols (***) and (**) represent statistical significance at p<0.001 and p<0.01, respectively. ![]()
Effect of SIL treatment on the status of lipid peroxidation in liver
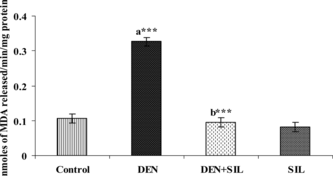
The effect of SIL treatment on the status of LPO in the liver tissue of control and experimental animals is presented in . DEN administration produced a 3-fold elevation in the levels of MDA, the oxidized products of lipid peroxidation. Treatment with SIL for 30 days after DEN administration decreased these levels of MDA significantly in group III rats when compared with group II rats.
Figure 3 igure 3 Effect of DEN and SIL on lipid peroxidation in liver tissue of experimental animals. Results are given as mean ± SE for six rats. Comparisons are made between: a, control rats (group I); b, DEN-treated rats (group II). The symbol (***) represents statistical significance at p<0.001. ![]()
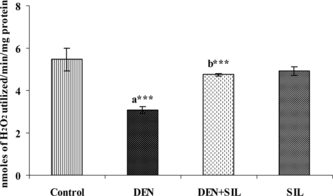
Effect of SIL treatment on antioxidant enzymes in liver
The activities of SOD and CAT in the liver during SIL treatment after DEN administration is depicted in and . There was a significant decrease in the activities of both SOD and CAT by up to 50% upon administration of DEN in the liver tissue. Treatment with SIL for 30 days after DEN administration restored the activities of SOD and CAT in the liver tissue to that of near normalcy.
Figure 4 Effect of DEN and SIL on the activity of SOD in liver tissue of experimental animals. Results are given as mean ± SE for six rats. Comparisons are made between: a, control rats (group I); b, DEN-treated rats (group II). The symbol (***) represents statistical significance at p<0.001. ![]()
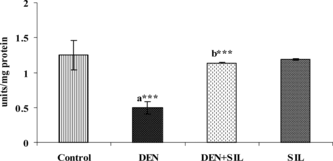
Figure 5 Figure 5Effect of DEN and SIL on the activity of CAT in liver tissue of experimental animals. Results are given as mean ± SE for six rats. Comparisons are made between: a, control rats (group I); b, DEN-treated rats (group II). The symbol (***) represents statistical significance at p<0.001. ![]()
Effect of SIL treatment on nonenzymic antioxidants in liver
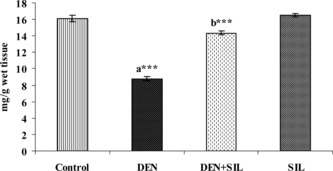
and show the level of vitamin C and vitamin E in the liver tissue of control and experimental rats. There was a significant decrease in the levels of these antioxidant vitamins upon administration of DEN in the liver tissue. Treatment with SIL for 30 days after DEN administration restored the levels of vitamin C and vitamin E in the liver tissue. Administration of SIL alone for a period of 30 days did not produce any significant alterations in any of the parameters investigated.
Figure 6 Figure 6 Effect of DEN and SIL on the levels of vitamin C in liver tissue of experimental animals. Results are given as mean ± SE for six rats. Comparisons are made between: a, control rats (group I); b, DEN-treated rats (group II). The symbol (***) represents statistical significance at p<0.001. ![]()
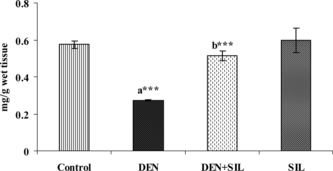
Figure 7 Effect of DEN and SIL on the levels of vitamin E in liver tissue of experimental animals. Results are given as mean ± SE for six rats. Comparisons are made between: a, control rats (group I); b, DEN-treated rats (group II). The symbol (***) represents statistical significance at p < 0.001. ![]()
Discussion
DEN is reported to be a well-established hepatotoxin and hepatocarcinogen (Goldsworthy & Hanigan, Citation1986). DEN-induced hepatocellular damage is confirmed after 30 days of administration of a single dose by the marked elevation in the levels of serum AST, ALT, ALP, and γ-GT, and a simultaneous fall in their levels in the liver tissue. Serum AST, ALT, ALP, and BIL are the most sensitive markers employed in the diagnosis of hepatic damage (Vasudevan & Sreekumari, Citation2001). Elevated levels of transaminases in serum observed in this study might be due to the release of these enzymes from the cytoplasm into the blood circulation rapidly after rupture of the plasma membrane and cellular damage caused by DEN and its metabolites (Sallie et al., Citation1991). γ-GT is a more sensitive indicator of hepatobiliary disease, and measurement of serum γ-GT is a frequently used parameter of liver diseases (Huseby & Ingebretsen, Citation1993). Increase in the levels of LDH has been reported in hemolytic anemia, hepatocellular necrosis, and hepatocellular carcinoma (Vasudevan & Sreekumari, Citation2001). The elevated levels of LDH and γ-GT observed in this study might be due to hepatic necrosis or premalignant hepatocellular lesions induced by DEN. Administration of SIL for 30 days significantly decreased these DEN-induced aberrations in the liver tissue as evidenced by restoration in the levels of these markers to near normal levels. This could be due to the protective effect elicited by SIL on the hepatocellular membranes, thus maintaining the membrane integrity and preventing further cellular damage. These observations are in accordance with that of El-Samaligy et al. (Citation2006) who observed a similar effect in rats for silymarin against CCl4-induced hepatotoxicity.
Lipid peroxidation is one of the main manifestations of oxidative damage initiated by reactive oxygen species (ROS), and it has been linked with impairment of membrane functioning, decreased fluidity, inactivation of membrane-bound receptors and enzymes, and increased nonspecific permeability to ions. Measurement of lipid peroxidation is therefore considered to be a convenient method to monitor oxidative membrane damage (Viani et al., Citation1991). In the current study, a 3-fold increase in the levels of LPO observed in liver tissue of DEN-administered rats is the consequence of oxidative stress caused by the metabolic activation of DEN resulting in peroxidative membrane damage. SIL treatment was found to offer a high degree of protection to the liver from peroxidative damage as noticed by a highly significant fall in LPO. This might be due to the effective quenching of free radicals by SIL, thereby protecting the liver from further oxidative damage.
Free radical scavenging enzymes like SOD and CAT protect the biological systems from oxidative stress. The SOD dismutates superoxide radicals (O2·−) into H2O2 and O2 (Fridovich, Citation1986). CAT further detoxifies H2O2 into H2O and O2 (Percy, Citation1984). Thus, SOD and CAT act mutually and constitute the enzymic antioxidative defense mechanism against reactive oxygen species (Bhattacharjee, Citation2006). DEN administration decreased the activities of these antioxidant enzymes in the liver tissue by about 50%. The decline in the levels of these enzymes in the current study could be attributed to the excessive utilization of these enzymes in inactivating the free radicals generated during the metabolism of DEN. Decrease in the levels of these key enzymes will lead to further deleterious effects due to the accumulation of superoxide radical and hydrogen peroxide. The reversal in the activities of these enzymes upon SIL administration is indicative of the fact that SIL is effective in preventing the accumulation of ROS, thereby preventing further oxidative damage. These findings are further substantiated by the studies of Batakov (Citation2001) who observed that SIL was able to reduce LPO and increase the levels of CAT in CCl4 hepatotoxicity induced rats.
Vitamin E and C act synergistically to scavenge the free radicals formed in the biological system. Vitamin C can protect cell membranes and lipoprotein particles from oxidative damage by regenerating the antioxidant form of vitamins and preventing the formation of excessive free radicals (Das, Citation1994). The decreased levels of these antioxidant vitamins observed during DEN administration might be due to the excessive utilization of these vitamins in scavenging the free radicals formed during the metabolism of DEN. The recoupment of vitamin C to near normal levels in SIL-treated rats was due to the potent antioxidant activity of SIL, which might have induced the regeneration of ascorbic acid. Vitamin E is a significant antioxidant that protects cells against free radical induced cytotoxicity and carcinogenesis (Beuttner, Citation1993). The elevation in the status of vitamin E during SIL treatment indicated the antioxidant and free radical scavenging potential of SIL. The protective effects of SIL observed in this study are in accordance with the investigations of Gonzalez-Correa et al. (Citation2002), who observed the antioxidant effect of SIL against oxidative stress in rat brain. Further, Zielinska-Przyjemska and Wiktorowicz (Citation2006) have also shown that silydianin, an important and major constituent of SIL, possesses excellent in vitro. free radical scavenging activity.
Conclusions
In conclusion, we observed that SIL offers excellent hepatoprotective and free radical scavenging activity against DEN-induced hepatocellular damage in rats. It prevents hepatocellular damage by virtue of its antioxidant activity and membrane stabilizing activity.
Acknowledgments
The authors wish to thank the UGC-UWPFE Project (No: HS 43) for the financial assistance provided for this study. We also thank Dr. Ram Raghubir, Scientist, CDRI, Lucknow, India, for his kind gift of silymarin for this study.
References
- Ahmed S, Rahman A, Mathur M, Athar M, Sultana S (2001): Antitumour promoting activity of Asteracantha longifolia. against experimental hepatocarcinogenesis in rats. Food Chem Toxicol l39: 19–28.
- Akintonwa DA (1985): The derivation of nitrosamines from some therapeutic amines in the human environment. Ecotocicol Environ Saf 9: 64–70.
- Barbisan LF, Scolastici C, Miyamoto M, Salvadori DMF, Ribeiro LR, daEira AF, deCamargo JLV (2003): Effects of crude extracts of Agaricus blazei. on DNA damage and on rat liver carcinogenesis induced by diethylnitrosamine. Gen Mol Res 2: 295–308.
- Batakov EA (2001): Effect of Silybum marianum. oil and legalon on lipid peroxidation and liver antioxidant systems in rats intoxicated with carbon tetrachloride. Eksp Klin Farmakol 64: 53–55.
- Belmenthal M (1998): The Complete German Commission E Monograph: Therapeutic Guide to Herbal Medicine. Austin, American Botanical Counciland Boston,Integrative Medicine Communications, p. 163.
- Beuttner GR (1993): The peeking order of free radicals and antioxidants: Lipid peroxidation, alpha-tocopherol and ascorbate. Arch Biochem Biophys 300: 535–543.
- Bhattacharjee R, Sil PC (2006): The protein fraction of Phyllanthus niruri. plays a protective role against acetaminophen induced hepatic disorder via its antioxidant properties. Phytother Res 20: 595–601.
- Choksi S, Patel SS, Saluja AK (2000): Silymarin—a promising herbal hepatoprotective drug. Ind Drugs 37: 566–569.
- Das S (1994): Vitamin E in the genesis and prevention of cancer. A review. Acta Oncol 33: 615–619.
- El-Samaligy MS, Afifi NN, Mahmoud EA (2006): Evaluation of hybrid liposomes-encapsulated silymarin regarding physical stability and in vivo. performance. Int J Pharmacy 319: 121–129.
- Feher J, Deak G, Muzes G, Lang I, Niederland V, Nekam K (1989): Liver protection action of silymarin therapy in chronic alcoholic liver diseases. Orv Hetil 130: 2723–2727.
- Flora K, Hahn M, Rosen H, Benner K (1998): Milk thistle (Silybum marianum.) for the therapy of liver diseases. Am J Gastroenterol 93: 139–143.
- Fraschini F, Demartini G, Esposti D (2002): Pharmacology of silymarin. Clin Drug Invest 22: 51–65.
- Fridovich I (1986): Superoxide dismutases. Adv Enzymol 58: 61–97.
- Goldsworthy TL, Hanigan MH (1986): Models of hepatocarcinogenesis in the rat—contrast and comparisons. CRC Crit Rev Toxicol 17: 61–89.
- Gonzalez-Correa JA, de la Cruz JP, Gordillo J, Urena I, Redondo L, Sanchez de la Cuesta F (2002): Effects of silymarin MZ-80 on hepatic oxidative stress in rats with biliary obstruction. Pharmacology 64: 18–27.
- Huseby NE, Ingebretsen OC (1993): The level of γ-glutamyltransferase in serum, effect of carbohydrate heterogeneity on clearance rate. Scand J Clin Lab Invest Suppl 215: 93–100.
- IARC (1972): Monograph on the Evaluation of Carcinogenic Risk of Chemicals to Man, Vol. 1. Lyon, International Agency for Research on Cancer, pp. 107–124.
- Jose JK, Kuttan R, Bhattacharaya RK (1998): Effect of Emblica officinalis. extract on hepatocarcinogenesis and carcinogen metabolism. J Clin Biochem Nutr 25: 31–39.
- King J (1965a): The phosphohydrolases—acid and alkaline phosphatases. In: Practical Clinical Enzymology. London, D. Van Nostrand Co Ltd., pp. 191–208.
- King J (1965b): The dehydrogenase (or) oxidoreductase—Lactate dehydrogenase. In: Practical Clinical Enzymology. London, D. Van Nostrand Co Ltd., pp. 83–93.
- Kren V, Walterova D (2005): Silybin and silymarin—new effects and applications. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 149: 29–41.
- Liao DJ, Blanck A, Eneroth P, Gustafsson JA, Hallstrom IP (2001): Diethylnitrosamine causes pituitary damage, disturbs hormone levels and reduces sexual dimorphism of certain liver functions in the rat. Environ Health Perspect 109: 943–947.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951): Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275.
- Malloy HT, Evelyn KA (1937): The determination of bilirubin with photoelectric colorimeter. J Biol Chem 119: 481–490.
- Marklund S, Marklund G (1974): Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47: 469–474.
- Muriel P, Garciapina T, Perez-Alvarez V, Mourelle M (1992): Silymarin protects against paracetamol induced lipid peroxidation and liver damage. J Appl Toxicol 12: 439–442.
- Muriel P, Mourelle M (1990): Prevention by silymarin of membrane alterations in acute CCl4 liver damage. J Appl Toxicol 10: 275–279.
- Ohkawa H, Ohishi N, Yagi K (1979): Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358.
- Omaye ST, Turnbull JD, Sauberlich HE (1979): Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. Methods Enzymol 62: 1–11.
- Percy ME (1984): Catalase: An old enzyme with a new role? Can J Biochem Cell Biol 62: 1006–1014.
- Pitot H, Campbell H, Maronpot R, Bawa N, Rizvi T, Xu YH, Sargent L, Dragan Y, Pyron M (1989): Critical parameters in the quantitation of the stages of initiation, promotion and progression in one model of hepatocarcinogenesis in the rat. Toxicol Pathol 17: 594–612.
- Rosalki SB, Rau D (1972): Serum γ-glutamyl transpeptidase activity in alcoholism. Clin Chim Acta 39: 41–47.
- Saller R, Meier R, Brignoli R (2001): The use of silymarin in the treatment of liver diseases. Drugs 61: 2035–2063.
- Sallie R, Tredger JM, Willam R (1991): Drugs and the liver. Biopharm Drug Dispos 12: 251–259.
- Sinha AK (1972): Colorimetric assay of catalase. Anal Biochem 47: 389–394.
- Varley H, Gowenlock AH, Bell M (1976): In: Practical Clinical Biochemistry, Vol. II, Hormones, Vitamins, Drugs and Poisons, 5th ed. London, William Heinemann Medical Books Ltd, pp. 222–223.
- Vasudevan DM, Sreekumari S (2001): In: Text Book of Biochemistry for Medical Students, 3rd ed. New Delhi, Jaypee Brothers Medical Publishers (P) Ltd, p. 271.
- Viani P, Cervato G, Fiorilli A, Cestaro B (1991): Age-related differences in synaptosomal peroxidative damage and membrane properties. J Neurochem 56: 253–258.
- Vitaglione P, Morisco F, Caporaso N, Fogliano V (2004): Dietary antioxidant compounds and liver health. Crit Rev Food Sci Nutr 44: 575–586.
- Wooten IDP (1964): In: Microanalysis in Medical Biochemistry. London, J & A Churchill Ltd, pp. 101–103.
- Yoshikawa M, Murakami T, Kishi A, Kageura T, Matsuda H (2001): Medicinal flowers III. Marigold (I): Hypoglycaemic, gastric emptying inhibitory and gastro protective principles and new oleane type triterpene oligoglycosides, calendasaponins A, B, C and D from Egyptian Calendula officinalis.. Chem Pharm Bull 49: 863–870.
- Zielinska-Przyjemska M, Wiktorowicz K (2006): An in vitro. study of the protective effect of the flavonoid silydianin against reactive oxygen species. Phytother Res 20: 115–119.