Abstract
The antidiarrheal effects of the aqueous extract of Punica granatum. L. (Punicaceae) peels were evaluated in rats. Studies were carried out on the isolated rat ileum, gastrointestinal motility in vivo., and on castor oil–induced diarrhea in rats. The results revealed that the extract exhibited a concentration-dependent inhibition of the spontaneous movement of the isolated rat ileum and attenuated acetylcholine-induced contractions. The extract (100, 200, 300, and 400 mg/kg) also caused a dose-dependent decrease of gastrointestinal transit and markedly protected rats against castor oil–induced diarrhea enteropooling. The intraperitoneal injection LD50 of the extract was found to be 1321 ± 15 mg/kg in mice. A preliminary phytochemical screening of the aqueous extract of Punica granatum. peels gave positive tests for tannins, flavonoids, and alkaloids. The results obtained showed that the aqueous extract of Punica granatum. peels may contain some biologically active principles that may be active against diarrhea, and this may be the basis for its traditional use for gastrointestinal disorders.
Introduction
Punica granatum. L. (Punicaceae) is a shrub, usually with multiple stems, that commonly grows 1.8–4.6 m tall. The deciduous leaves are shiny and about 7.6 cm long. Punica granatum. has orange-red, trumpet-shaped flowers with ruffled petals. The flowers are about 5 cm long, often double, and are produced over a long period in summer. The fruit is globose, 5–7.6 cm in diameter, and shiny reddish or yellowish green when mature. The fruit is technically a berry. It is filled with crunchy seeds, each of which is encased in a juicy, somewhat acidic pulp that is itself enclosed in a membranous skin (Polunin & Huxley, Citation1987). Punica granatum. grows well in warm areas and is cultivated throughout Jordan, where it is popularly known as “roman.” Almost all parts of this plant are used in traditional medicine for the treatment of various ailments. Bark and rind of the fruit are used in dysentery, diarrhea, piles, bronchitis, to reduce the risk of cardiovascular disease, and as an anthelmintic (Polunin & Stainton, Citation1985; Morton, Citation1987). Methanol extract of Punica granatum. seed and dried peels has antidiarrheal activity and wound-healing activity, respectively (Das et al., Citation1999; Murthy et al., Citation2004). In a recent study, Punica granatum. juice was found to reduce cholesterol oxidation by almost half and reduce the retention of disproportionate low-density lipoprotein (LDL) cholesterol (Singh et al., Citation2002). Punica granatum. rind extract has been shown to have gastroprotective activity through its antioxidant mechanism (Ajaikumar et al., Citation2005). A breast cancer chemopreventive property of Punica granatum. fruit extracts has been found in a mouse mammary organ culture (Mehta & Lansky, Citation2004). Other studies have also evaluated the Punica granatum. extract and established it to be effective as an antibacterial and anticandidal agent (Navarro et al., Citation1996; Rani & Khullar, 2004).
Several compounds have been isolated from Punica granatum. such as tannins, punicalagin, ellagic acid, hydroquinone pyridinium, delphinidin, cyanidin, and pelargonidin. Punicalagin and tannin have antiproliferative and antioxidant activities (Noda et al., Citation2002; Schmidt et al., 2005; Seeram et al., Citation2005).
Despite the relatively wide use of this plant in popular medicine in Jordan and other Middle East countries for its antidiarrheal properties, surprisingly no research has been carried out to examine the antidiarrheal activity of its peels extract. The current study thus was carried out to evaluate the antidiarrheal effect of aqueous extract of Punica granatum. peels using various validated models and to determine if the folk medicinal use has a scientifically justified basis.
Materials and Methods
Plant material
Peels of Punica granatum. were collected from the Wadi Alseer area of Amman (Jordan) in 2005. The plant material was identified and authenticated by a plant taxonomist at the Hashemite University Herbarium (Zarka, Jordan), where a voucher specimen has been deposited for future reference.
Preparation of aqueous extract
To obtain the aqueous extract of Punica granatum. peels, 150 g of the ground, air-dried peel was boiled in 3000 mL of distilled water for 15 min with continuous stirring. The resultant solution was filtered through a filter paper. The filtrate was completely evaporated under reduced pressure at 60°C. An equivalent of 2 g of powder was obtained from 150 g of dried peels. Solutions were prepared by dissolving the resultant powder in physiologic salt solution (PSS). PSS was prepared daily and had the following composition (mM): 118 NaCl, 4.7 KCl, 25 NaHCO3, 1 NaH2PO4·H2O, 0.5 Na2HPO4, 11.1 glucose, 2.5 MgCl2·6H2O, and 2.5 CaCl2·2H2O. The pH of stock solution was adjusted to 7.4.
Phytochemical analysis
The aqueous extract of the plant was subjected to qualitative chemical screening for the identification of the tannins, alkaloids, and flavonoids using standard procedures (Trease & Evans, Citation2001).
Test for tannins
The aqueous extract (1 mL) was mixed with 10 mL of distilled water and filtered. Ferric chloride reagent (3 drops) was added to the filtrate. A blue-black or green precipitate confirmed the presence of gallic tannins or catechol tannins, respectively.
Test for alkaloids
The aqueous extract (0.2 mL) was stirred and placed in 1% aqueous hydrochloric acid (5 mL) on a steam bath. Then, 1 mL of the filtrate was treated with Mayer's reagent (3 drops) while another portion was similarly treated with Dragendorff's reagent. Turbidity or precipitation with these reagents was considered as evidence for the presence of alkaloids.
Test for flavonoids
A portion of the aqueous extract (2 mL) was heated, and metallic magnesium and concentrated hydrochloric acid (5 drops) were added. A red or orange coloration indicated the presence of flavonoids.
Animals
Adult albino rats of either sex, weighting between 150 and 200 g, were used. Animals were provided with the standard animal feed and tap water. Food was withdrawn 18 h before antidiarrheal experiments but water was allowed. All animals used in this study were cared for in accordance with guidelines of the Hashemite University Animal Care Committee.
Antidiarrheal test
Rats were housed in six cages containing six rats each. Rats in groups A, B, C, and D received 100, 200, 300, and 400 mg/kg, respectively, the doses were selected on a trial basis, and those in group E received 5 mg/kg of diphenoxylate as positive control. The sixth group (group F), which served as a control, received PSS only. All administrations were intraperitoneal. The rats were then housed singly in cages lined with white blotting paper. One hour after the above treatments, the rats were given 1 mL of castor oil orally. The rats were observed at time intervals, up to 5 h after the castor oil administration, for the presence of diarrhea. Diarrhea, for the purpose of this study, was taken to mean watery (wet), unformed stool. The number of wet droppings was counted every hour for a period of 5 h.
Anti-enteropooling test
Intraluminal fluid accumulation was determined by the method of Robert et al. (Citation1976). Fasted rats were divided into five groups of six animals each. Group A received PSS intraperitoneally and served as control. Groups B, C, D, and E received 100, 200, 300, and 400 mg/kg intraperitoneally of the plant extract, respectively. The above treatments were given 1 h before administration of 1 mL of castor oil orally. Two hours later, the rats were sacrificed, and the small intestine was ligated both at the pyloric sphincter and at the ileocecal junctions and dissected out. The small intestine was weighed. The intestinal contents were collected by milking into a graduated tube and the volume was measured. The intestines were reweighed and the differences between full and empty intestines were calculated.
Gastrointestinal motility
Charcoal food (0.5 mL of 5 g of activated charcoal suspended in 50 mL PSS) was given to six groups of six animals. In groups A, B, C, and D, the charcoal food was administrated to animals intragastrically 60 min after the intraperitoneal injection of aqueous extract of Punica granatum. (100, 200, 300, and 400 mg/kg, respectively). Group E was treated with 1 mg/kg of atropine sulfate instead of the aqueous extract. Controls (group F) were treated with PSS before receiving the charcoal food.
The animals were sacrificed after 60 min of charcoal administration, and the small intestine, from the pylorus to the cecum, was rapidly removed and laid out on white filter paper for inspection and measurement of the distances traversed by the front of the charcoal food. This distance was calculated as a percentage of the whole intestine length.
Ileal preparation and contractility test on ileum
Rats were lightly anesthetized with ether and were sacrificed by a sharp blow to the head, and the abdomen was opened. Segments of the ileum about 1–2 cm long were removed and dissected free of adhering mesentery. The lumen was flushed with PSS. The tissue was mounted in a 10 mL organ bath containing PSS at 37 ± 1°C and aerated with air (95% O2 and 5% CO2). A tension of 1 g was applied. The responses were recorded isosmetrically on a minigraph (Lafayette Instrument Company) after a 60-min equilibrium period during which the PSS was changed every 15 min as a precaution against tissue metabolites (Abu Ghalyun et al., 1997). After an equilibrium period, the effects of acetylcholine (3 × 10−10 to 1 × 10−8M) and aqueous extract of Punica granatum. peel was investigated. The responses of the ileum to aqueous extract of Punica granatum. peel were expressed as a percentage of the maximum relaxation to papaverine (10−3 M), which was added at the end of the experiment.
Acute toxicity test
The LD50 value of the aqueous extract and its 95% confidence limits were determined using the method of Lorke (1983). Different doses of extract were injected intraperitoneally into groups of 18 mice. The number of deaths was counted at 48 h after treatment.
Chemicals
All chemicals used in this study were of analytical grades and were purchased from Gainland Chemical Company (UK), BDH Laboratory Supplies (UK), and Sigma (St. Louis, MO, USA).
Statistical analysis
The values were expressed as the mean ± S. E. A Student's t.-test was used for the evaluation of data and p < 0.05 accepted as significant. The concentration producing 50% of the maximum response (EC50 or IC50) was obtained by the best visual fit from the plot of the individual experiments.
Results
Effect on castor oil test
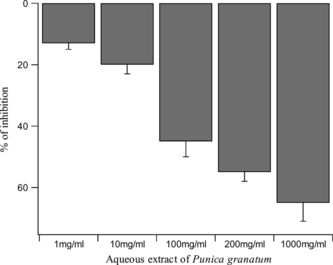
Sixty minutes after administration of castor oil, diarrhea was apparent in all the animals of control group for the next 4 h. This was largely eliminated by the intraperitoneal injection of loperamide. The effect of the aqueous extract was not as potent as the standard drug (loperamide) used, but it was dose-related (). The IC50 value was 174 ± 4 mg/kg.
Table 1.. Effect of Punica granatum. peels extract on 1 mL castor oil–induced diarrhea in rats.
Effect on castor oil–induced enteropooling
Castor oil caused accumulation of water and electrolytes in intestinal loop. Treatment with the Punica granatum. extract (100, 200, 300, and 400 mg/kg) produced a significant and dose-dependent reduction in intestinal weight and volume with IC50 values of 148 ± 3 mg/kg and 141 ± 5 mg/kg, respectively ().
Table 2.. Effect of Punica granatum. peels extract on 1 ml castor oil-induced enteropooling in rats.
Effect on intestinal motility
The extract (100, 200, 300, and 400 mg/kg) significantly reduced the gastrointestinal distance traveled by the charcoal in animals, compared with the control (). The IC50 value was 138 ± 4 mg/kg. The highest inhibition of intestinal transit was obtained with atropine sulfate.
Table 3.. Effect of Punica granatum. peels extract on intestinal motility expressed as distance traveled by the charcoal food as percent of the total intestinal length.
Effects on the rat ileum
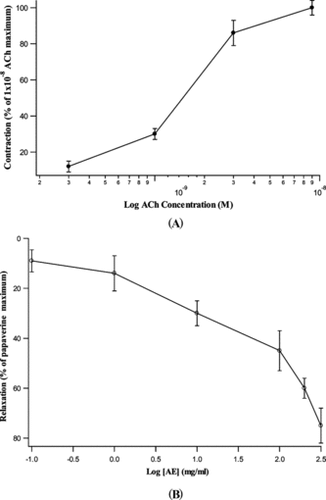
Acetylcholine (3 × 10−10 to 1 × 10−8M) caused a concentration-dependent contraction of the rat ileum, and the extract (0.1–450 mg/mL) produced a dose-dependent inhibition of the spontaneous contraction of ileum (). The EC50 and IC50 of acetylcholine and aqueous extract on the ileum were 1.7 × 10−9 ± 0.2 × 10−9 M and 20 ± 1.4 mg/mL, respectively. The extract (1–1000 mg/mL) attenuated acetylcholine (5 × 10−9 M) induced contractions of the rat ileum concentration dependently with an IC50 value of 30 ± 2 mg/kg ().
Acute toxicity
The preliminary acute toxicity test in mice showed that the aqueous extract at doses below 500 mg/kg was sublethal and doses above 1800 mg/kg were completely lethal. The intraperitoneal injection LD50 was 1321 ± 15 mg/kg.
Phytochemical analysis
The extract gave positive tests for tannins, alkaloids, and flavonoids ().
Table 4.. Chemical constituents of aqueous extract of Punica granatum. peels.
Discussion
Our results revealed that, in a dose-dependent manner (100, 200, 300, and 400 mg/kg body weight), the aqueous extract of Punica granatum. peels appears to contain substance(s) that reduced diarrhea by inhibiting intestinal motility and intestinal fluid accumulation. The inhibitory effect of the aqueous extract of Punica granatum. peels justifies the use of the plant in folk medicine and its use as a nonspecific antidiarrheal agent. The extract meets some of the criteria for acceptance of a drug as an antidiarrheal (Akah, Citation1988). These criteria include inhibition of the production of wet or unformed feces in animals and inhibition of gastrointestinal propulsive action.
The effect of the extract was similar to loperamide, which is at present one of the most efficacious and widely employed antidiarrheal drugs; loperamide effectively antagonized diarrhea induced by castor oil (Karim & Adaikan, Citation1997) and prostaglandins (Niemegeers et al., Citation1974). The therapeutic effect of loperamide is believed to be due to antimotility and antisecretory properties (Couper, Citation1987). From this study, it is likely that the extract may mediate its effects through similar mechanisms.
Castor oil is reported to produce changes in intestinal mucosal membrane permeability to electrolytes and water and thus produces diarrhea (Galves et al., Citation1993; Bruton, Citation2001). The antidiarrheal activity of Punica granatum. peels extract could be due to several mechanisms: (1) an increase in the water and NaCl reabsorption, (2) reduced mucosal secretion, and (3) inhibition of prostaglandin release from intestinal mucosa.
The extract may increase the reabsorption of water and NaCl by decreasing intestinal motility. This is supported by observation that the intestinal motility was significantly reduced in treated animals compared with control.
Tannates are known to reduce mucosal secretion and make the intestinal mucosa more resistant (Scalbert, Citation1991; Tripathi, Citation1994). The presence of such water-soluble polyphenols in the aqueous extract of Punica granatum. peels (as observed in this study) may mediate the antidiarrheal property of Punica granatum. peels.
Liberation of ricinoleic acid by castor oil results in irritation and inflammation of intestinal mucosa. Inflammation leads to the release of prostaglandins. Prostaglandins may increase mucosal secretion (Pierce et al., Citation1971). Flavonoids and alkaloids are known for inhibiting release of autocoids and prostaglandins, thereby inhibiting secretion induced by castor oil (Vimala et al., Citation1997; Veiga et al., Citation2001). Phytochemical analysis of the aqueous extract of Punica granatum. peels revealed the presence of flavonoids and alkaloids. These constituents may mediate the antidiarrheal property of Punica granatum. peels extract.
The extract similarly inhibited spontaneous and agonist-induced contractions of the rat ileum and markedly reduced the intestinal motility. These effects might contribute to the observed antidiarrheal activity. Antimicrobial activity of the extract (Rani & Khullar, 2004), even though not proven, may contribute to the antidiarrheal effect of the extract during infectious diarrhea.
Punica granatum. peelsaqueous extract caused a concentration-dependent relaxation of rat ileal smooth muscle. The contractions of smooth muscles are known to depend on the concentration of intracellular Ca+2 (Jiang & Stephens, Citation1994; Ganitkevich et al., Citation2002). Because plant extract caused an inhibition of the contractions of the ileum, this implies that this extract decreased the cytosolic calcium, either by inhibiting Ca+2 influx or by inhibiting Ca+2 release from intracellular stores, or both. The relaxant effect could also have resulted from other mechanisms such as a decrease in the sensitivity of contractile apparatus to existing concentrations of Ca+2 and/or inhibition of the binding of Ca+2 to the contractile proteins (Savineau & Marthan, Citation1997). Further studies are required to ascertain the precise mechanism of action of aqueous extract on ileal smooth muscles.
Acknowledgment
This work was supported by a grant of the Deanship for Scientific Research, Hashemite University.
References
- Abu Ghalyun Y, Masalmeh A, Al-khalil S (1997): Effects of allocryptopine, an alkaloid isolated from Glaucium arabicum. on rat isolated ileum and urinary bladder. Gen Pharmacol 4: 713–716.
- Ajaikumar KB, Asheef M, Babu BH, Padikkala J (2005): The inhibition of gastric mucosal injury by Punica granatum. L. (pomegranate) methanolic extract. J Ethnopharmacol 96: 171–176.
- Akah P (1988): Purgative potentials of Euphorbia heterophylla.. Fitoterapia IX (1): 45–48.
- Bruton L (2001): Agents affecting gastrointestinal water flux and motility; Emesis and antiemetics; Bile acids and pancreatic enzymes. In: The Pharmacological Basis of Therapeutics, 10th ed. New York, McGraw-Hill, pp. 155–173.
- Couper IM (1987): Opioid action on the intestine: The importance of the intestinal mucosa. Life Sci 41: 917–925.
- Das AK, Mandal SC, Banerjee SK, Sinha S, Das J, Saha BP, Pal M (1999): Studies on antidiarrheal activity of Punica granatum. seed extract in rats. J Ethnopharmacol 68: 205–208.
- Galves J, Zavzuelo A, Crespo M, Lorente M, Ocete M, Jimenez J (1993): Antidiarrheic activity of Euphorbia hirta. extract and isolation of an active flavonoid constituent. Planta Med 59: 333–336.
- Ganitkevich V, Hasse V, Pfitzer G (2002): Ca2+-dependent and Ca2+-independent regulation of smooth muscle contraction. J Muscle Res Cell Motil 23: 47–52.
- Jiang H, Stephens NL (1994): Calcium and smooth muscle contraction. Mol Cell Biochem 135: 1–9.
- Karim SM, Adaikan PG (1997): The effect of loperamide on prostaglandin induced diarrhea in rat and man. Prostaglandins 13: 321–331.
- Lorke D (1983): A new approach to practical acute toxicity testing. Arch Toxicol 54: 275–287
- Mehta R, Lansky EP (2004): Breast cancer chemopreventive properties of pomegranate (Punica granatum.) fruit extracts in a mouse mammary organ culture. Eur J Cancer Prev 13: 345–348.
- Morton J (1987): Pomegranate. In: Fruits of Warm Climates. Miami, Julia F. Morton, pp. 352–355.
- Murthy KN, Reddy VK, Veigas JM, Murthy UD (2004): Study on wound healing activity of Punica granatum. peel. J Med Food 7: 256–259.
- Navarro V, Villarreal ML, Rojas G, Lozoya X (1996): Antimicrobial evaluation of some plants used in Mexican traditional medicine for the treatment of infectious diseases. J Ethnopharmacol 53: 143–147.
- Niemegeers CJ, Lenaerts FM, Janssen PA (1974): Loperamide (R 18 553), a novel type of antidiarrheal agent. Part 1: In vivo. oral pharmacology and acute toxicity. Comparison with morphine, codeine, diphenoxylate and difenoxine. Arzneimittelforschung 24: 1633–1636.
- Noda Y, Kaneyuki T, Mori A, Packer L (2002): Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Delphinidin, cyanidin, and pelargonidin. J Agric Food Chem 50: 166–171.
- Ofuji K, Hara H, Sukamoto T, Yamashita S (1998): Effects of an antidiarrheica containing an extract from Geranium. herb on astringent action and short-circuit current across jejunal mucosa. Nippon Yakurigaku Zassh 111: 265–275.
- Pierce N, Carpenter C, Elliot H, Greenough W (1971): Effects of prostaglandins, theophylline, and cholera exotoxin upon transmucosal water and electrolyte movement in the canine jejunum. Gastroenterology 60: 22–32.
- Polunin O, Huxley A (1987): Pomegranate. In: Flowers of the Mediterranean. Hogarth Press, pp. 54–57.
- Polunin O, Stainton A (1985): Pomegranate. In: Flowers of the Himalayas. New York, Oxford University Press, pp. 532–534.
- Rani P, Khullar N (2004): Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multi-drug resistant Salmonella typhi.. Phytother Res 18: 670–673.
- Robert A, Nezamis J, Lancaster C, Hanchar A, Klepper M (1976): Enteropooling assay: A test for diarrhea produced by prostaglandins. Prostaglandins 11: 809–828.
- Savineau J, Marthan R (1997): Modulation of the calcium sensitivity of the smooth muscle contractile apparatus: Molecular mechanisms, pharmacological and pathophysiological implications. Fundam Clin Pharmacol 11: 289–299.
- Scalbert A (1991): Antimicrobial properties tannins. Phytochemistry 30: 3875–3883.
- Schmidt A, Mordhorst T, Nieger M (2005): Investigation of a betainic alkaloid from Punica granatum.. Nat Prod Res 19: 541–546.
- Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, Heber D (2005): In vitro. antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem 16: 360–367.
- Singh RP, Chidambara Murthy KN, Jayaprakasha GK (2002): Studies on the antioxidant activity of pomegranate (Punica granatum.) peel and seed extracts using in vitro. models. J Agric Food Chem 50: 81–86.
- Trease G, Evans M (2001): Pharmacopoeial and related drugs of biological origin. In: A Textbook of Pharmacognosy, 15th ed. London, WB Saunders, pp. 262–270.
- Tripathi K (1994): Essential of Medical Pharmacology. New Delhi, Jaypee Brothers Medical Publishers, p. 187.
- Veiga V, Zunino L, Calixto J, Patitucci M, Pinto A (2001): Phytochemical and antioedematogenic studies of commercial copaiba oils available in Brazil. Phytother Res 15: 476–480.
- Vimala R, Nagarajan S, Alam M, Susan T, Joy S (1997): Antiinflammatory and antipyretic activity of Michelia champaca. Linn. (white variety), Ixora brachiata. Roxb. and Rhynchosia cana. (Willd.) D.C. flower extract. J Exp Biol 35: 1310–1314.