Abstract
The cytotoxic potential of ethanol extracts from Peperomia elongata. H. B. & K. (Piperaceae) were evaluated against human cancer cell lines by the MTT method. The samples considered cytotoxic were tested for antimitotic activity with the sea urchin egg development test and for hemolytic activity using mice erythrocytes. The extracts from leaves (hexane), stems (ethanol, hexane, hexane:AcOEt, AcOEt, and MeOH:H2O insoluble), and roots (R4) presented potential cytotoxic action. The stems extracts showed the highest toxicity in all tumor cell lines tested, with an IC50 ≤ 9.0 µg/mL for ethanol extract, IC50 ≤ 11.6 µg/mL for MeOH:H2O insoluble, IC50 ≤ 7.3 µg/mL for hexane extract, IC50 ≤ 11.4 µg/mL for hexane: AcOEt, and IC50 ≤ 16.2 µg/mL for AcOEt extract. All extracts considered cytotoxic for tumoral cell lines presented antimitotic activity. The samples from roots (R4) and stems (ethanol, MeOH:H2O insoluble, and hexane extract from leaves) were found to possess lytic activity in mice erythrocytes but in higher doses (> 125 µg/mL). Further studies for the isolation and identification of the active principles of these extracts should be undertaken.
Introduction
Cancer represents a serious human health problem despite progress in understanding its biology and pharmacology. The main problem is that cancer is not one disease but a group of diseases affecting different organs and systems in the body. There are cogent reasons why cancer is difficult to cure: one is that the differences between normal and neoplastic human cells are mostly quantitative; another is that by their very nature, cancer cells have eluded or overcome the immune surveillance systems of the body (Cocco, Citation2003). Normal homeostatic mechanisms that regulate many aspects of cell behavior are upset in cancer cells (Yarnold, Citation1996). The tumor progression phenomenon is related to sequential appearance of subpopulation of cells that differ with respect to several phenotypic attributes, such as invasiveness, rate of growth, metastatic ability, karyotype, hormonal responsiveness, and susceptibility to antineoplastic drugs (Cotran et al., Citation1994).
The development of potent and effective antineoplastic drugs has become one of the most intensely pursued goals of contemporary medicinal chemistry. Within the Piperaceae, the genus Peperomia. has received little phytochemical attention, in contrast with the extensive studies of Piper. compounds (Parmar et al., Citation1997; Alécio et al., Citation1998; Baldoqui et al., Citation1999; Mbah et al., Citation2002; Navickiene et al., Citation2000; Seeram et al., Citation2000; Danelutte et al., Citation2003; Martins et al., Citation2003; Lago et al., Citation2004Citation2005). The biological activities of the genus Peperomia. and its species have limited information in the literature (Cheng et al., Citation2003). Common constituents from genus Peperomia. are phenylpropanoids, benzopyrans, chromones, prenylated quinone, and acylcyclohexane-1,3-dione (Cheng et al., Citation2003; Salazar et al., Citation2005).
Many Peperomia. metabolites possess interesting biological activities (Seeram et al., Citation2000). Some Peperomia. species have been used as folk medicine, for example, P. japonica. Makino has been used for the treatment of malignant tumors (Tanaka et al., Citation1998; Mbah et al., Citation2002). In this context, we have embarked in exploiting the biological properties of Peperomia elongata. H. B. & K. extracts on tumor cell lines, developing embryos of sea urchins and mouse erythrocytes.
Materials and Methods
Plant material
Leaves, stems, and roots of Peperomia elongata. were collected in August 2003 from plants maintained at the Instituto de Química (USP). The specimen was identified by Dr. Elsie F. Guimarães (Instituto de Pesquisas Jardim Botanico do Rio de Janeiro), and a voucher specimen has been deposited (Kato-0568).
Extraction
Dried leaves (34 g), stems (124 g), and roots (13 g) were milled and extracted three-times with ethanol at room temperature and the extract concentrated under reduced pressure yielding a brownish, viscous residue. The ethanol extracts from leaves (yield 19%) and stems (yield 9%) were solubilized in MeOH:H2O (2:1) (yield 11% and 25%, respectively) and then solvent-partitioned in hexane (yield 30% and 11%, respectively), followed by further extraction of the water layer with hexane:AcOEt (2:8) (yield 18% and 7%, respectively), and further partitioning with AcOEt (yield 10% and 6%, respectively). Part of the stems ethanol extract did not solubilize in MeOH:H2O, and then it was denominated as MeOH:H2O insoluble extract (yield 42%). The roots extract (yield 10%) was partitioned between MeOH and hexane [R1: MeOH insoluble (yield 3%); R3: hexane insoluble (yield 24%); and R4: hexane soluble (yield 14%)]. The extracts were evaporated by rotary evaporator to produce solids or viscous oils.
MTT assay screening
The cytotoxicity of the extracts was tested against B-16 (murine melanoma), HCT-8 (human colon carcinoma), MCF-7 (human breast carcinoma), CEM and HL-60 (human leukemias) tumor cell lines (National Cancer Institute, Bethesda, MD, USA). Cells were cultured in RPMI-1640 medium, supplemented with 10% fetal calf serum, 2 mM glutamine, 100 µg/mL streptomycin, and 100 U/mL penicillin at 37°C with 5% CO2. For experiments, cells were plated in 96-well plates (0.7 × 105 cells/well for adherent cells or 0.5 × 106 cells/well for suspended cells in 100 µl medium). In a first set of experiments, the extracts (125 µg/mL) dissolved in DMSO (1%) were added to each well after 24 h and then incubated for 3 days (72 h). Control groups received the same amount of DMSO. Doxorubicin (0.58 µg/mL) was employed as positive control. Growth of tumor cells was quantified by the ability of living cells to reduce the yellow dye 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H.-tetrazolium bromide (MTT) to a blue formazan product (Mosmann, Citation1983). At the end of a 72 h incubation period, the medium in each well was replaced by fresh medium (200 µL) containing 0.5 mg/mL MTT. Three hours later, the formazan product of MTT reduction was dissolved in DMSO, and absorbance was measured using a multiplate reader (Spectra Count, Packard, Ontario, Canada). Drug effect was quantified as the percentage of control absorbance of reduced dye at 540 nm. The extracts that exhibited a growth inhibitory effect greater than 90% in this prescreen were selected for a second experiment in order to determine the IC50 values. In these experiments, the extract concentration ranged from 2 to 125 µg/mL. Extract was considered cytotoxic when IC50 ≤ 20 µg/mL in at least two cell lines tested.
Assay of sea urchin
Adult sea urchins (Lytechinus variegatus.) were collected at Lagoinha Beach, on the northeastern coast of Brazil. The gamete elimination was induced by injecting 3.0 mL of 0.5 M KCl into the sea urchin's coelomic cavity via the periostomial membrane. The eggs were washed twice using filtered seawater to remove the jelly coat surrounding the cells. Concentrated sperm was collected with a Pasteur pipette and maintained at a low temperature (4±1°C) until the moment of fertilization. For fertilization, 1 mL of a sperm suspension (0.05 mL concentrated sperm in 2.45 mL filtered seawater) was added to every 50 mL of egg solution. The assay was carried out in 24-multiwell plates. Each well received 1 mL of fertilized egg suspension. The extracts were added immediately after fecundation (within 2 min) to achieve concentrations of 10, 30, 100, 300, and 1000 µg/mL in a final volume of 2 mL. At appropriate intervals, aliquots of 200 µL were fixed in the same volume of 10% formaldehyde to obtain first and third cleavages and blastulae. One hundred eggs or embryos were counted for each concentration of test substance to obtain the percentage of normal cells. Extract antimitotic activity was noted when IC50 ≤ 100 µg/mL in at least one of the three phases of sea urchin egg development (Costa-Lotufo et al.., Citation2005).
Hemolytic assay
The test was performed in 96-well plates following the method described (Costa-Lotufo et al., Citation2005). Each well received 100 µL of 0.85% NaCl solution containing 10 mM CaCl2. The first well was the negative control that contained only the vehicle (distilled water or DMSO 10%), and, in the second well, 100 µL of test substance that was diluted in half was added. The extracts were tested at concentrations ranging from 7.94 to 1000 µg/mL. The serial dilution continued until the 11th well. The last well received 20 µL of 0.1% Triton X-100 (in 0.85% saline) to obtain 100% hemolysis (positive control). Then, each well received 100 µL of a 2% suspension of mouse erythrocytes in 0.85% saline containing 10 mM CaCl2. After incubation at room temperature for 30 min and centrifugation, the supernatant was removed, and the liberated hemoglobin was measured spectroscopically as absorbance at 540 nm.
Statistical analysis
Data are presented as means±SEM. The IC50 or EC50 and their 95% confidence intervals were obtained by nonlinear regression using the GraphPad program (Intuitive Software for Science, San Diego, CA, USA).
Results and Discussion
In this study, the antimitotic potential of Peperomia elongata. extracts was estimated as the ability of these extracts to inhibit tumor cell line growth and sea urchin egg development. Their hemolytic activities on mouse erythrocytes were also evaluated.
The first 17 samples have been evaluated in primary cytotoxic assay at the concentration of 125 µg/mL against HL-60 (human leukemia), B-16 (murine melanoma), HCT-8 (human colon cancer), and MCF-7 (breast) cellas. The extracts with high activity, that is, exhibiting a growth inhibitory effect higher than 90%, were retested to establish their IC50 values. Among the selected 15 samples, only seven were considered cytotoxic, that is, with IC50 values lower than 20 µg/mL. Among the samples from roots, only R4 was considered cytotoxic and, among samples from leaves, only hexane was cytotoxic (). The stem extracts showed the highest toxicity in all tumor cell lines tested, with an IC50 ≤ 9.0 µg/mL for ethanol extract, IC50 ≤ 11.6 µg/mL for MeOH:H2O insoluble, IC50 ≤ 7.3 µg/mL for hexane extract, IC50 ≤ 11.4 µg/mL for hexane:AcOEt, and IC50 ≤ 16.2 µg/mL for AcOEt extract ().
Table 1.. IC50 (µg/mL) values of Peperomica elongata. extracts against different tumor cell lines.
Antimitotic activity was then evaluated for these seven samples using the sea urchin egg development assay. All the seven samples were also active in this model (). The extract from root (R4) was active on all three phases of embryonic development. The extracts from stems (ethanol, MeOH:H2O insoluble, hexane, hexane:AcOEt) were active in all the three phases of sea urchin egg development, but the AcOEt was not active in the first cleavage. The extract from leaves (hexane) was active only in the third cleavage (). The sea urchin egg development possessed some peculiarities, which allow a preliminary interpretation on mode of action. The sea urchin cell cycle is highly abbreviated, essentially cycling from S (DNA synthesis) to M (mitosis) and S with no G1 and a relatively short G2 phase (Jacobs & Wilson, Citation1986). The inhibition of the first cleavage of the sea urchin egg development is related to DNA and/or protein synthesis or microtubule assembling, once RNA synthesis is very slow or absent after fertilization (Gross et al., Citation1964; Brandshort, Citation1985).
Table 2.. Antimitotic activity of Peperomia elongata. extracts on sea urchin (Lytechinus variegatus.) egg developement.
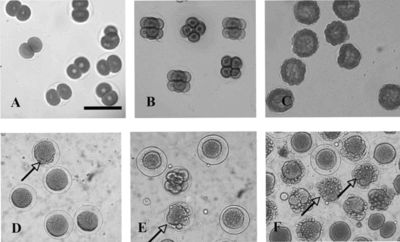
The observation of treated sea urchin embryos under the microscope revealed undivided cells in which the plasmatic membrane was damaged but with the fecundation membrane intact () in a sample treated with extracts from stems (hexane), suggesting an action in membrane. At doses near the IC50 levels, we observed normal aspects of the sea urchin egg development inhibition, suggesting that in this model, the mechanism of cytotoxicity is not a result of membrane damage itself, but of other more specific processes.
Figure 1 Photomicrographs showing the effect of the hexane fraction of the ethanol extract obtained from the stems of Peperomia elongata. on the sea urchin egg development: (A, B, C) control; (D, E, F) treated with 100 µg/mL at first and third cleavages and blastulae stages, respectively. Horizontal bar = 100 µm. The arrows indicate cell destruction.
The hemolysis assay was performed with the seven samples considered cytotoxic for the tumor cell lines tested, in order to verify whether the observed cytotoxicity is related to membrane disruption. Samples from stems (hexane:AcOEt, AcOEt) and leaves (hexane) did not present lytic activity against mouse erythrocytes (). Samples from roots (R4) and stems (ethanol, MeOH:H2O insoluble, hexane) presented lytic activity against mouse erythrocytes () but in higher doses (> 125 µg/mL), suggesting that the mechanism of cytotoxicity at the IC50 levels is not a result of membrane damage. The lowest EC50 value was from leaves (hexane), the sample that presented an action on the membrane of sea urchin eggs ().
Table 3.. Hemolytic activity of Peperomia elongata. extracts on mouse erythrocyte (2%).
Among Peperomia elongata. extracts analyzed, the stems samples demonstrated high growth inhibition activity in all cell lines. Between extracts assayed, those from leaves (hexane), stems (ethanol, hexane, hexane:AcOEt, AcOEt, and MeOH:H2O insoluble), and roots (R4) presented potential cytotoxic action. The extracts considered cytotoxic were those that displayed IC50 ≤ 20 µg/mL against at least two cell lines. The samples considered cytotoxic for tumor cell lines presented antimitotic action against sea urchin eggs, too. Samples from roots (R4) and stems (ethanol, MeOH:H2O insoluble, hexane) presented lytic activity against mouse erythrocytes () but in higher doses (> 125 µg/mL) than the IC50 levels for the MTT assay and for the sea urchin egg assay. The results obtained from MTT method (cytotoxic and antiproliferative activity) for Peperomia elongata. extracts should be further evaluated looking at isolation of bioactive compounds.
Acknowledgments
This work was funded by grants provided by FAPESP and CNPq/PADCT. This work was also supported by the State of São Paulo Research Foundation (FAPESP) within the BIOTA/FAPESP–The Biodiversity Virtual Institute Program (www.biotasp.org.br), CNPq, FINEP, and Banco do Nordeste. The technical assistance of Silvana França is gratefully acknowledged.
References
- Alécio AC, Bolzani VS, Young MCM, Kato MJ, Furlan M (1998): Antifungal amides from Piper hispidum.. J Nat Prod 61: 637–639.
- Baldoqui DC, Kato MJ, Cavalheiro AJ, Bolzani VS, Young MCM, Furlan M (1999): New chromene and prenylated benzoic acid from Piper aduncum.. Phytochemistry 51: 899–902.
- Brandshort PB (1985): Informational content of the echinoderm egg. In: Browder LW, ed. Developmental Biology a Comprehensive Synthesis. Oogenesis. New York, Plenum Press, pp. 525–576.
- Cheng MJ, Lee SJ, Chang YY, Wu SH, Tsai IL, Jayaprakasan B, Chen IS (2003): Chemical and cytotoxic constituents from Peperomia sui.. Phytochemistry 63: 603–608.
- Cocco MT, Congiu C, Onnis V (2003): Synthesis and in vitro. antitumoral activity of new N.-phenyl-3-pyrrolecarbothioamides. Bioorg Med Chem 11: 495–503.
- Costa-Lotufo LV, Khan, MTH, Ather A, Wilke DV, Jimenez PC, Pessoa C, Moraes MEA, Moraes MO (2005): Sudies of the anticancer potential of plants used in Bangladeshi folk medicine. J Ethnopharmacol 99: 21–30.
- Cotran RS, Kumar V, Robbins SL (1994): Neoplasia. In: Cotran RS, Kumar V, Robbins SL, eds., ROBBINS: Pathologic Basis of Disease, 5th Ed.. Philadelphia, W. B. Saunders, pp. 241–303.
- Danelutte AP, Lago JHG, Young MCM, Kato MJ (2003): Antifungal flavanones and prenylated hydroquinones from Piper crassinervium. Kunth. Phytochemistry 64: 555–559.
- Jacobs RS, Wilson L (1986): Fertilized sea urchin egg as a model for detecting cell division inhibitors. In: Aszalor A, ed. Modern Analysis of Antibiotics. New York, Marcel Dekker, pp. 481–493.
- Gross PR, Malkin JL, Moyer WA (1964): Templates for the first proteins of embryonic development. Proc Natl Acad Sci USA 51: 407–414.
- Lago JHG, Ramos CS, Casanova DCC, Morandim AA, Bergamo DCB, Cavalheiro AJ, Bolzani VS, Furlan M, Guilharães EF, Young MCM, Kato MJ (2004): Benzoic acid derivatives from Piper. species and their fungitoxic activity against Cladosporium cladosporioides. and C. sphaerospermum.. J Nat Prod 67: 1783–1788.
- Lago, JHG, Tanizaki T, Young MCM, Guimarâes EF, Kato MJ (2005): Antifungal piperolides from Piper malacophyllum. (Prels) C. DC.). J Braz Chem Soc 16: 153–156.
- Martins RC, Lago JHG, Kato MJ (2003). Trypanocidal tetrahydrofuran lignans from Piper solmsianum.. Phytochemistry 64: 667–670.
- Mbah JA, Tchuendem MHK, Tane P, Sterner O (2002): Two chromones from Peperomia vulcanica.. Phytochemistry 60: 799–801.
- Mosmann T (1983): Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 16: 55–63.
- Navickiene HMD, Alécio AC, Kato MJ, Bolzani VS, Young MC, Cavalheiro AJ, Furlan M (2000): Antifungal amides from Piper hispidum. and Piper tuberculatum.. Phytochemistry 55: 621–626.
- Parmar SV, Jain SC, Bisht KS, Jain R, Taneja P, Jha A, Tyagi OD, Prasad AK, Wengel J, Olsen CE, Boll PM (1997): Phytochemistry of the genus Piper.. Phytochemitry 46: 597–673.
- Salazar KJM, Paredes GED, Lluncor LR, Young MCM, Kato MJ (2005): Chromenes of polyketide origin in Peperomia villipetiola.. Phytochemistry 66: 573–579.
- Seeram NP, Lewis AW, Jacons H, Nair MG, McLean S, Reynolds WF (2000): Proctoriones A-C: 2-Acylcyclohexane-1,3-dione derivatives from Peperomia proctorii.. J Nat Prod 63: 399–402.
- Tanaka T, Asai F, Iinuma M (1998). Phenolic compounds from Peperomia obtusifolia.. Phytochemistry 49: 229–232.
- Yarnold JR (1996): What are cancer genes and how do they upset cell behaviour? In: Yarnold JR, Stratton M, McMillan TJ, eds., Molecular Biology for Oncologists. London, Chapman & Hall, pp. 3–15.