Abstract
The antioxidative potential of ethanol extracts of Hypericum triquetrifolium. Turra (Hypericaceae) and Hypericum scabroides. Robson & Poulter (Hypericaceae) were investigated for the first time using 1,1-diphenyl-2-picrylhydrazyl (DPPH), metal chelating, reducing power, hydroxyl radical, total antioxidant activity, and lipid peroxidation inhibition assays. Both extracts tested were found to be highly active in the DPPH radical scavenging assay. The IC50 values of H. triquetrifolium. (HT) and H. scabroides. (HS) in the DPPH radical scavenging assay were 39.0 and 33.8 μ g/mL, respectively. The amounts of total phenolic compounds were also determined, and total phenolic content of 1 mg H. triquetrifolium. and H. scabroides. ethanol extracts were equivalent to 267 and 333 μ g gallic acid. Metal chelating ability was found to be low compared with EDTA. Both ethanol extracts of Hypericum. species exhibited a high reducing power, suggesting that extracts had strong electron-donating capacity. The degradation of deoxyribose by hydroxyl radicals was shown to be inhibited by Hypericum. extracts, acting mainly by scavenging hydroxyl radicals rather than as chelators of iron ions. Total antioxidant activity of ethanol extracts of HT and HS were tested by using ferric thiocyanate (FTC) and thiobarbituric acid (TBA) methods. Antioxidative activities of both extracts were found to be comparable with vitamin E. Moreover, both extracts showed notable capacity to suppress Fe2 +-induced lipid peroxidation in rat brain homogenate. The results obtained in the current study indicate that ethanol extracts of HS and HT are a potential source of natural antioxidants.
Introduction
Oxidative modifications of DNA, proteins, lipid, and small cellular molecules by reactive oxygen species (ROS) play a role in a wide range of common diseases and age-related degenerative conditions (Borek, Citation1993). These include cardiovascular disease, inflamatory conditions, and neurodegenerative disease such as Alzheimer's, mutations, and cancer (Block et al., Citation1992; Hertog et al., Citation1993; Richardson, Citation1993; Byres & Guerrero, Citation1995; Kaur & Kapoor, Citation2001; Landbo & Meyer, Citation2001). Furthermore, antioxidants are also believed to play a cardinal role in the oxidative deterioration of cosmetics, foodstuffs, and pharmaceutical preparations. There is an increasing interest in natural antioxidants, namely polyphenols, present in medicinal and dietary plants that might help prevent oxidative damage. The antioxidant activity of several plant materials has recently been described (Shimada et al., Citation1992; Liu & Ng, Citation2000; Schinella, 2000; Bridi et al., Citation2001; Dasgupta & De, Citation2004; David et al., Citation2004; Gulcin et al., 2004; Kosar et al., 2005), and a number of plant products, including polyphenols, flavonoids, and terpenes, exert an antioxidant action.
The genus Hypericum. (Hypericaceae), which contains more than 400 species, is widespread throughout the world and well represented in the Mediterranean and the Near East area (Robson et al., Citation1986). The genus Hypericum. has been receiving attention as a medicinal plant because a number of species have been found to be effective in the treatment of burns and gastrointestinal diseases (Baytop, Citation1984; Mukherjee et al., Citation2000). Its antibacterial, antifungal, and antioxidant activities have been investigated by many researchers (Decosterd et al., Citation1991; Pistelli et al., Citation2000; Conforti et al., Citation2002; Rabanal et al., Citation2002; Schwob et al., Citation2002). Also, Hypericum. species contain a variety of compounds such as flavonoids (Chung et al., Citation1997; Dias et al., Citation1998; Wu et al., Citation1998a), xanthones (Gunatilaka et al., Citation1979; Rath et al., Citation1996; Wu et al., Citation1998b), hyperforin derivates (Decosterd et al., Citation1989; Trifunuvic et al., 1998), essential oils (Cakir et al., Citation1997), and fatty acids (Ozen & Başhan, 2002). Among these species, Hypericum perforatum. L., also known as St. John's wort, has been extensively examined, and several studies have been published concerning Hypericum perforatum. L. activities, which have shown that this species has antidepressant, anxiety, antiviral, wound healing, and antimicrobial activities (Sakar and Tamer, Citation1990; Butterweck et al., Citation1997, Citation2000, Citation2002; Barnes et al., Citation2001; Flausino et al., Citation2002). Hypericum perforatum. extract contains flavonoids such as rutin, quercetin, and quercitrin, which demonstrated a free radical scavenging activity in a model of autooxidation of rat cerebral membranes (Saija et al., Citation1995). In recent years, the consumption of Hypericum perforatum. (St. John's wort) derived products has increased dramatically, and currently it is one of the most consumed medicinal plants throughout the world.
There are about 80 species of Hypericum. genus in Turkey, and these species have long been used for the treatment of external wound and gastric ulcer, and also as sedative, antiseptic, and antispasmodic in folk medicine (Baytop, Citation1984). The essential oils and various extracts of H. triquetrifolium. and H. scabroides. have been examined for their anti-inflammatory and antimicrobial activities (Sokmen et al., Citation1999; Ozturk et al., Citation2002; Kizil et al., Citation2004; Toker et al., Citation2006). Antioxidant activity of H. hyssopifolium. L. was also examined by Cakir et al. (Citation2003), and six phenolic compounds have been isolated from the ethyl acetate fraction of a methanol extract of H. hyssopifolium..
Synthetic antioxidants such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), which are commonly used in processed foods, are known to have toxic and carcinogenic effects on human health (Ito et al., Citation1986; Lean & Mohamed, Citation1999; Matthaus, Citation2002). Therefore, screening of plant species to identify new antioxidants has become critically important in recent years. There are so far no reports related to antioxidant activity of ethanol extracts of H. triquetrifolium. and H. scabroides.. The purpose of this study was to determine the antioxidant capacity and phenolic contents of ethanol extracts of H. triquetrifolium. Turra (Hypericaceae) and H. scabroides. Robson & Poulter (Hypericaceae).
Materials and Methods
Collection of plant material
H. triquetrifolium. Turra and H. scabroides. Robson & Poulter were collected in Mardin and şırnak, respectively, in Southeastern Turkey in June 2005. Voucher specimens have been deposited at the herbarium of the Department of Biology, Faculty of Science and Art, Dicle University (voucher no. DUF-2512-a and DUF-2512-b, respectively). They were identified by Dr. A. Selçuk Ertekin from the same institution.
Preparation of crude extract
Aerial parts (stems, leaves, and flowers) were dried for 10 days at room temperature. Aerial parts of H. triquetrifolium. (198 g) and H. scabroides. (175 g) were ground in an electric blender and then incubated in a glass flask with 2000 mL ethanol (70%) for 3 days under a magnetic stirrer. The crude ethanol extracts of H. triquetrifolium. (23 g), with a purple color, and H. scabroides. (16 g), with a brown color, were obtained and kept in dark glass bottles at 4°C until use.
Scavenging activity of DPPH radical
Free radical scavenging activity of ethanol extracts of H. triquetrifolium. (HT) and H. scabroides. (HS) was measured by 1,1-diphenyl-2-picrylhydrazyl (DPPH; Sigma Aldrich, Steinheim, Germany) using the method of Shimada et al. (Citation1992). Briefly, a 0.1 mM solution of DPPH in ethanol was prepared. Then, 1 mL of this solution was incubated with varying concentrations of ethanol extracts of HT and HS (5–500 μ g/mL). The reaction mixtures were then shaken well and incubated for 30 min in dark at room temperature. The absorbance of the resulting solution was read at 517 nm against a blank. The radical scavenging activity was calculated as follows:
Ascorbic acid, butylated hydroxytoluene (BHT), and α-tocopherol (Sigma Aldrich) were used as positive controls. IC50 values were calculated by linear regresion analysis using Prism version 2.0 software.
Determination of total phenolic compounds
The content of total phenolic compounds in ethanol extracts of HT and HS was determined using Folin-Ciocalteu reagent according to the method of Singleton et al. (Citation1999). Crude ethanol extracts (40 μ L) of HT and HS (1 mg/mL) were mixed with 200 μ L Folin-Ciocalteu reagent (Sigma Aldrich) and 1160 μ L of distilled water, followed by 600 μ L 20% sodium carbonate (Na2CO3) 3 min later. The mixture was shaken for 2 h at room temperature, and absorbance was measured at 765 nm. All tests were performed in triplicate. Gallic acid (Sigma Aldrich) was used as a standard. The concentration of total phenolic compounds in H. triquetrifolium and H. scabroides. was determined as μ g of gallic acid equivalents per 1 mg of extract using the following equation obtained from a standard gallic acid graph (R2 = 0.9878).
Determination of reducing power
The reducing power of H. triquetrifolium. and H. scabroides. was determined according to the method of Oyaizu (Citation1986). Different concentrations of ethanol extracts of HT and HS (50–250 μ g) in 1 mL distilled water were mixed with phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 mL, 1%). The mixture was incubated at 50°C for 20 min. A portion (2.5 mL) of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3000 rpm for 10 min. The upper layer of the solution (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%). The absorbance was measured at 700 nm. A higher absorbance indicated a higher reducing power. Butylated hydroxytoluene and α-tocopherol were used as standards.
Metal ion chelating assay
The chelating of ferrous ions by the ethanol extracts of HT and HS was estimated by the method of Dinis et al. (Citation1994). Briefly, extracts (12.5–62.5 μ g/mL) were added to a solution of 2 mM FeCl2 (0.05 mL). The reaction was initiated by the addition of 5 mM ferrozine (0.2 mL), and the mixture was shaken vigorously and left standing at room temperature for 10 min. Absorbance of the solution was then measured spectrophotometrically at 562 nm. EDTA (50–500 μ g) was used as a positive control. All test and analyses were run in triplicate and averaged. The percentage of inhibition of ferrozine-Fe2 + complex formation was calculated using the formula given below:
where A.0 is the absorbance of the control, and A.1 is the absorbance in the presence of samples of extracts or standards. The control does not contain FeCl2 or ferrozine, complex formation molecules.
Determination of inhibitory effect on deoxyribose degradation
The reaction mixture, containing ethanol extracts of HT and HS (10–100 μ g/mL), was incubated with deoxyribose (10 mM), H2O2 (50 mM), FeCl3 (10 μ M), EDTA (1 mM), and ascorbic acid (10 mM) in potassium phosphate buffer (50 mM, pH 7.4) for 60 min at 37°C (Halliwell et al., Citation1987). Then, reaction was terminated by adding 1 mL 10% TBA (1% w/v) and 1 mL TCA (2% w/v), and heating the tubes in a boiling water bath for 15 min. The contents were cooled and the absorbance of the mixture was measured at 532 nm against regeant blank. Decreased absorbance of the reaction mixture indicated decreased oxidation of deoxribose.
where A.c. is the absorbance of the control, and A.s. is the absorbance in the presence of samples of extracts or standards.
Antioxidant activity in linoleic acid system
Ferric thiocyanate (FTC) method
The method of ferric thiocyanate was followed from Kikuzaki and Nakatani (Citation1993), which was slightly modified by Mitsuda et al. (1967) and Osawa and Namiki (Citation1981). The FTC method was used to determine the amount of peroxide at the initial state of lipid peroxidation. The peroxide reacts with ferrous chloride (FeCl2) to form a reddish ferric choloride (FeCl3) pigment. In this method, the concentration of peroxide decreases as the antioxidant activity increases. A mixture of 4 mg of each sample was placed in 4 mL absolute ethanol (Merck, Darmstadt, Germany), 4.1 mg of 2.52% linoleic acid (Sigma Aldrich Gmbh, Sternheim, Germany) in absolute ethanol, 8 mL 0.05 M phosphate buffer (pH 7.0), and 3.9 mL water was placed in a vial with a screw cap, and then placed in an oven at 40°C in the dark. To 0.1 mL of this solution, 9.7 mL of 75% ethanol and 0.1 mL 30% ammonium thiocyanate (Sigma) were added. Exactly 3 min after the addition of 0.1 mL 0.02 M ferrous chloride in 3.5% hydrochloric acid (HCl) to the reaction mixture, the absorbance was measured at 500 nm every 24 h until the absorbance of the control reached maximum. The control and standard were subjected to the same procedures as the sample, except that for the control, only the solvent was added, and for the standard, 4 mg sample was replaced with 4 mg vitamin E.
Thiobarbituric acid (TBA) method
The method of Ottolenghi (Citation1959) was used to determine the TBA values of the samples. The formation of malondialdehyde is the basis for the well-known TBA method used for evaluating the extent of lipid peroxidation. At low pH and high temperature (100°C), malondialdehyde binds TBA to form a red complex that can be measured at 532 nm. The increased amount of the red pigment formed correlates with the oxidative rancidity of the lipid. Trichloroacetic acid (20%) (2 mL) and 2 mL TBA aqueous solution were added to 1 mL sample solution prepared as in the FTC procedure, and incubated in a similar manner. The mixture was then placed in a boiling water bath for 10 min. After cooling, it was centrifuged at 3000 rpm for 20 min, and the absorbance of the supernatant was measured at 532 nm.
Antioxidant activity was determined as follows:
All total antioxidant data are the average of three replicates.
Lipid peroxidation in rat brain homogenate
The inhibition of lipid peroxidation of rat brain homogenate was assayed according to the method described by Ng et al. (Citation2000) with some modifications. Brain tissues obtained from female Wistar Albino rats weighing 150 g were homogenized with 120 mM KCl, 50 mM phosphate buffer, pH 7.4 (1:10 w/v), and centrifuged at 800 × g. for 10 min. An aliquot (100 μ L) of supernatant was mixed with 200 μ L ethanol extracts of HT and HS (0.25–2.0 mg/mL) followed by addition of 100 μ L 10 mM FeSO4 and 100 μ L 0.1 mm ascorbic acid. The mixture was incubated at 37°C for 1 h. The reaction was terminated by adding 500 μ L trichloroacetic acid (TCA; 28%) followed by 380 μ L TBA (2%) with heating at 100°C for 20 min. After this, samples were cooled on ice and then centrifuged 1000 × g. for 10 min, and the absorbance of the supernatant was measured at 532 nm. α-Tocopherol was used as a standard, and the inhibition percentage of lipid peroxidation of the sample was calculated by the following equation.
where A.c. is the absorbance of the control, and A.s. is the absorbance in the presence of samples of extracts or standard.
Statistical analyses
All analyses were performed in triplicate. The parameter values were all expressed as the mean ± standard deviation (SD). Significant differences among the groups were determined by one-way ANOVA using the SPSS 12.0 program. The results were considered significant if the value of p was less than 0.05.
Results
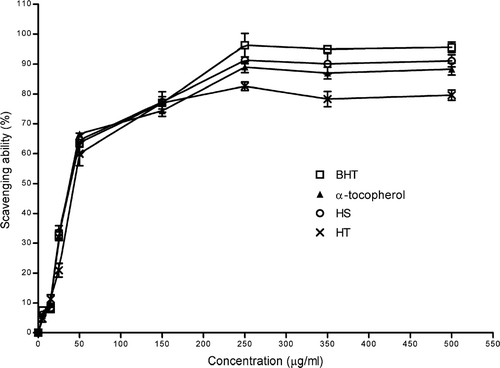
Antioxidants have an important role in preventing a variety of diseases and aging because they inhibit or delay the oxidation process by blocking the initiation or propagation of oxidizing chain reactions (Ames et al., Citation1993; Storz, Citation2005). In the current study, antioxidant activity of crude ethanol extracts of HT and HS were investigated using different antioxidant tests. The free radical scavenging effect of ethanol extracts of HT and HS was measured by DPPH assays. DPPH is a useful reagent for investigating free radical scavenging activities of phenolic compounds. The reduction of DPPH absorbtion is indicative of the capacity of the extract to scavenge free radicals, independently of any enzymatic activity (Lebeau et al., Citation2000). shows the DPPH radical scavenging activity of ethanol extracts of Hypericum. species and standards (α-tocopherol and BHT) at varying concentrations. The ethanol extracts of HT and HS and standards showed high radical scavenging activity at 250 μ g/mL. At this concentration, the scavenging effects were 96.3%, 91.3%, 89.0%, and 82.6% for BHT, ethanol extract of HS, α-tocopherol, and ethanol extract of HT, respectively. The scavenging effect of ethanol extracts of HS, HT, and standards rapidly increased from 5 to 50 μ g/mL, and reached a maximum level at 250 μ g/mL, and then leveled off with a slight decrease. The IC50 values of α-tocopherol, BHT, HS, and HT in the DPPH radical scavenging assay were 32.6, 37.9, 33.7, and 39.0 μ g/mL, respectively. No statistically significant difference was found between the ethanol extracts of Hypericum. species and standards (p > 0.05).
Figure 1 Scavenging effect of ethanol extracts of HS and HT on 1,1-diphenyl-2-picrylhydrazyl radicals. Each value is expressed as mean ± SD (n = 3).
The amount of total phenolic compounds was investigated in the ethanol extracts of HT and HS. Phenols are very important plant constituents because of their scavenging ability which is due to their hydroxyl groups (Diplock, Citation1997). The total amount of phenolic compounds in the plant extracts was determined as micrograms of gallic acid equivalent by using an equation that was obtained from standard gallic acid graph. HT and HS ethanol extracts (1 mg each) were equivalent to 267 and 333 μ g gallic acid, respectively.
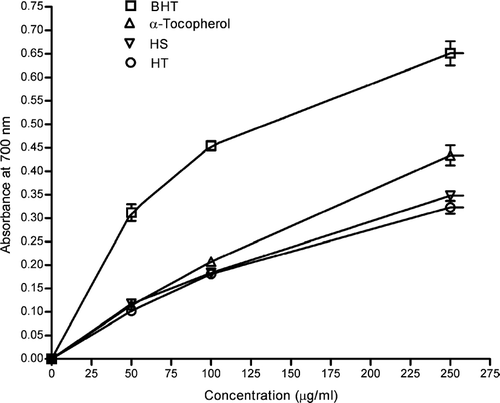
The reducing ability of a compound generally depends on the presence of reductants (Pin-Der-Duh, Citation1998), which have exhibited antioxidative potential by breaking the free radical chain, donating a hydrogen atom (Gordon, Citation1990). The presence of reductants in the extracts causes the reduction of the Fe3 +-ferricyanide complex to the ferrous form. Therefore, the Fe2 + can be monitored by measuring the formation of Perl's Prussian blue at 700 nm. shows reducing capacities of ethanol extracts of HT and HS compared with α-tocopherol and BHT. The reducing power of ethanol extracts of HT, HS, and standards increased with increasing concentration of samples. The reducing power of BHT was relatively more pronounced than those of α-tocopherol and ethanol extracts of HT and HS. At all tested concentrations, BHT showed higher activities than α-tocopherol and ethanol extracts of HT and HS. These differences were statistically significant (p < 0.05). Although reductive capability of α-tocopherol was higher than that of ethanol extracts of HS and HT, the differences were not statistically significant. The reducing ability of ethanol extracts of HS and HT were dose-dependent and significantly higher than the control.
Figure 2 Reducing power of ethanol extracts of HS and HT. Each value is expressed as mean ± SD (n = 3).
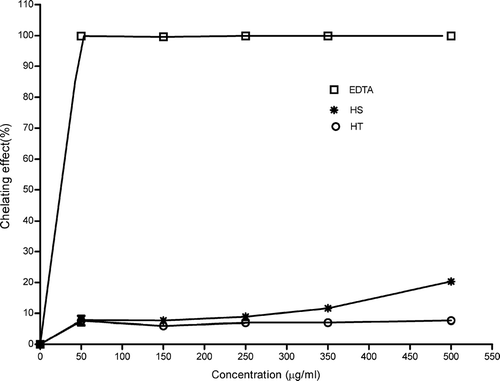
Ferrozine can quantitatively form complexes with Fe2 +, but in the presence of ion chelating agents, the complex formation is disrupted, resulting in a decrease in the red color of the complex. Ethanol extracts of both Hypericum. species showed very low ferrous iron chelating ability (). EDTA showed very strong chelating ability. The chelating effect of EDTA at all concentrations was approximately 100%. Ethanol extracts of HS showed relatively higher activity when compared with the that obtained from ethanol extracts of HT. The chelating effect of ethanol extract of HS started to increase at a concentration of 500 μ g/mL, and this increase was significantly higher (p < 0.05) than that of the ethanol extract of HT. At that concentration, chelating ability of HS and HT was 20.3% and 7.7%, respectively. The chelating effect of ethanol extract of HS was dose-dependent.
Figure 3 Chelating effect of ethanol extracts of HS, HT, and EDTA. Each value is expressed as mean ± SD (n = 3).
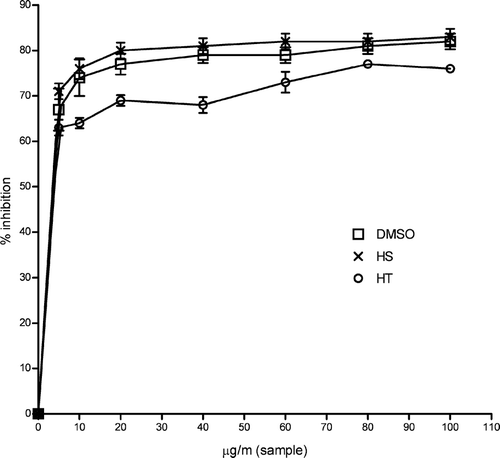
Hydroxyl radicals are the major active oxygen species causing lipid oxidation and enormous biological damage (Aurand et al., Citation1977). Ferric-EDTA was incubated with H2O2 and ascorbic acid at pH 7.4. Hydroxyl radicals were formed and were detected by their ability to degrade 2-deoxyribose into fragments that formed a pink chromogen upon heating with TBA at low pH (Halliwell et al., Citation1987; Aruoma et al., Citation1989). Ethanol extracts of Hypericum. species and the reference compound DMSO were added to the reaction mixture, and they removed hydroxyl radicals from the sugar and prevented their degradation. Hydroxyl radical scavenging activity was measured by studying the competition between deoxyribose and samples for hydroxyl radical generated by the Fe3 +-ascorbate-EDTA-H2O2 system (Fenton reaction) according to the method of Kunchandy and Rao (Citation1990). shows ethanol extracts of Hypericum. species were able to reduce DNA damage at all concentrations. Inhibition of deoxyribose oxidation of DMSO, HS, and HT increased rapidly at 5 μ g/mL. The maximum inhibition ability by DMSO and HS were 82% and 83% at 100 mg/mL, respectively. HT showed 77% maximum inhibition ability on deoxyribose oxidation at 80 mg/mL. Inhibition by both extracts was found to be statistically significant relative to control (p < 0.05). Ethanol extract of HS and DMSO standard also exhibited very similar inhibition activities on deoxyribose oxidation.
Figure 4 Effect of ethanol extracts of HS and HT on deoxyribose degradation assay. Each value is expressed as mean ± SD (n = 3).
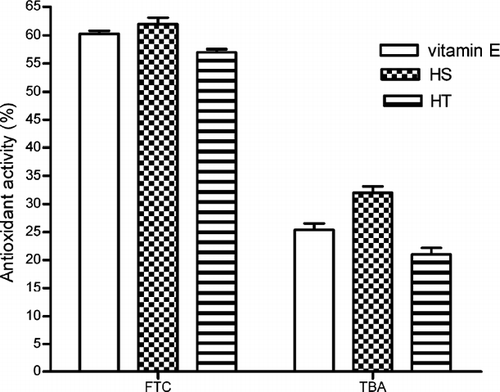
The total antioxidant activity of ethanol extracts of HT and HS were determined by peroxidation of linoleic acid using the FTC and TBA method. During linoleic acid peroxidation, peroxides were formed, and these compounds oxidized Fe2 + to Fe3 +. The Fe3 + ion formed a complex with SCN−, which had a maximum absorbance at 500 nm. Thus, a high absorbance value was an indication of high peroxide formation during the emulsion incubation. As shown in , the absorbance of the control at 500 nm increased to a maximal value of 0.95 on day 14, whereas vitamin E and ethanol extracts of HT and HS increased to 0.38, 0.36, and 0.41, respectively, on the same day, and these differences were found statistically significant relative to control (p < 0.05). shows the total antioxidant activity of ethanol extracts of HT and HS by FTC and TBA methods. Ethanol extract of H. scabroides. (62%) had the highest antioxidant activity, followed by vitamin E (60%) and ethanol extracts of HT. (57%). Based on the TBA method, ethanol extract of HS (32%) had the highest antioxidant activity, followed by vitamin E (25%) and ethanol extracts of HT (21%). No significant difference was found between the the total antioxidant activity of both ethanol extracts of Hypericum. species and vitamin E in both methods.
Figure 5 Absorbance value of ethanol extracts HS and HL in the linoleic acid emulsion using FTC method. Each value is expressed as mean ± SD (n = 3).
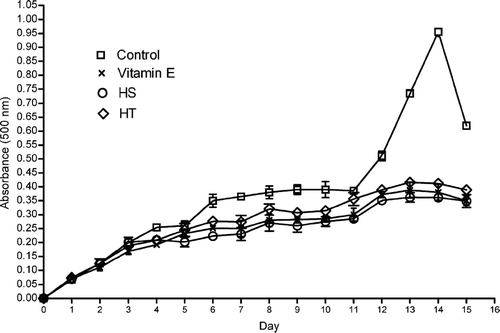
Figure 6 The total antioxidant activity of ethanol extracts of HS and HT by using FTC and TBA method. Each value is expressed as mean ± SD (n = 3).
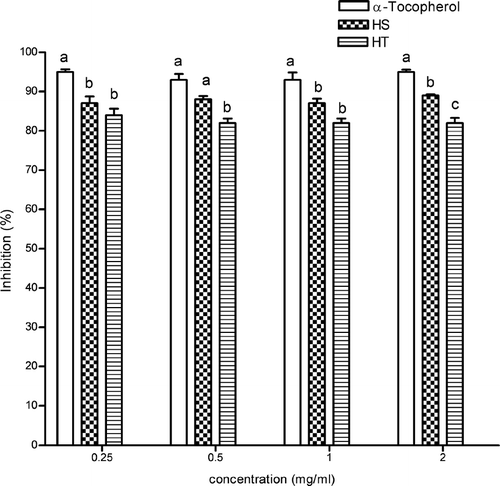
The antioxidant activities of both Hypericum. extracts were also evaluated by quantifying the ability of different concentrations of plant extracts to supress iron (Fe2 +)-induced lipid peroxidation in rat brain homogenates. shows that both Hypericum. extracts inhibited thiobarbiturie acid reactive substances (TBARS) formation in a concentration-independent manner. In all concentrations, α-tocopherol inhibits TBARS production better than ethanol extracts of HS and HT. The extracts also showed inhibition of peroxidation effect in all concentrations. At 0.5 and 2 mg/mL, ethanol extract of HS showed a greater increase than the ethanol extract of HT (p < 0.05). Both extract inhibition values were found to be statistically significant relative to the control (p < 0.05).
Figure 7 Effect of ethanol extracts of HS and HT on Fe2 +-induced lipid peroxidation in rat liver homogenates. Each value is expressed as mean ± SD (n = 3). Means with different letters differ significantly, p < 0.05. The values sharing common letters are not significantly different, p > 0.05.
Discussion and Conclusions
There are many reports that support the use of antioxidant supplementation in reducing the level of oxidative stress and in slowing or preventing the development of complications associated with diseases (Maxwell & Lip, Citation1997; Rose et al., Citation1982; Tandon & Gupta, Citation2005). Many synthetic antioxidant components have also shown toxic or mutagenic effects, which have shifted attention toward the naturally occurring antioxidants. Numerous plant constituents have been proved to show free radical scavenging or antioxidant activity (Aruoma & Cuppett, Citation1997; Sundararajan et al., Citation2006). In this respect, flavonoids and other polyphenolic compounds have received greatest attention.
Plant tissues contain a network of compounds that control the level of reactive oxygen species, including phenolic compounds, vitamins C and E, glutathione, and several enzymes. Phenolic compounds widely distributed in the natural plant tissues include flavonoids, tannins, hydroxycinnamate esters, and lignins (Rice-Evans et al., Citation1997). Furthermore, interest in employing antioxidants from natural sources to increase the shelf life of foods is considerably enhanced by consumer preference for natural ingredients and concerns about the toxic effects of synthetic antioxidants (De Lenardis & Macciala, 2003; Farag, Citation2003; Schwarz, Citation2001; Tang, Citation2001). The Hypericum. family seems to be a rich source of plant species containing large amounts of phenolic acids, so it is considered to be a promising source of natural antioxidants (Conforti et al., Citation2002; Silva, Citation2005).
In the current study, the antioxidant activities of ethanol extracts of HT and HS were determined by using different antioxidant tests. DPPH is a stable nitrogen-centered free radical the color of which changes from violet to yellow upon reduction by either the process of hydrogen or electron donating. Substances that are able to perform this reaction can be considered as antioxidants and therefore radical scavengers (Brand-Williams et al., Citation1995). Ethanol extracts of HS and HT showed a concentration dependent scavenging of DPPH, which may be attributable to its hydrogen-donating ability. Based on IC50 values, the highest DPPH radical scavenging effect was detected in ethanol extract of HT (IC50 = 39.0) followed by BHT (IC50 = 37.9), ethanol extract of HS (IC50 = 33.8), and α-tocopherol (IC50 = 32.6). It has been reported that the antioxidant activity of plant materials was well correlated with the content of their phenolic compounds (Emmon et al., Citation1999; Gao et al., Citation2000; Cheung et al., Citation2003). Estimation of total phenolics using the Folin-Ciocalteu reagent and gallic acid as a standard revealed that both Hypericum. species are a rich source of polyphenol. Similar to DPPH radical scavenging activity, ethanol extract of HS exhibited a higher level of phenolic compounds than ethanol extract of HT.
It has also been reported that reducing power is associated with antioxidant activity and may serve as a significant reflection of the antioxidant activity (Yen & Duh, Citation1993; Chang, et al., Citation2002). Ethanol extracts of HS and HT exhibited comparatively similar reducing power as α-tocopherol, suggesting that both Hypericum. species had strong electron-donating capacity. However, results based on chelating ability of ferrous ions show that ethanol extracts of HS and HT were not good chelating agents. The scavenging effect of extracts of HS and HT on hydroxyl radicals was also assayed by measuring the effect on the 2-deoxyribose degradation produced by reaction of Fe3 + with ascorbate in the presence of EDTA. The results obtain from this assay suggest that both Hypericum. extracts were moderate scavengers of hydroxyl radicals.
Antioxidants are known to alleviate oxidative stress, which is generally perceived as one of the major causes for the accumulation of mutations in the genome. Peroxide is gradually decomposed to lower molecular compounds during the oxidation process, and these compounds were here measured by FTC method and TBA methods. The amount of peroxide at the primary stage of linoleic acid peroxidation was measured by the FTC method, whereas TBA method measures at the secondary stage. Total antioxidant activity of ethanol extracts of HS and HT was determined by using FTC and TBA methods. Total antioxidant activity of the FTC method was compared with the TBA method, and the activity of antioxidant for FTC method is higher than for the TBA method. This may indicate that the amount of peroxide in the initial state of lipid peroxidation is greater than the amount of peroxide in the secondary stage. Furthermore, the secondary product such as malondialdehyde is not stable for a period of time. It will turn into alcohol and acid, which cannot be detected by spectrophotometer (Ottolenghi, Citation1959).
The extent of lipid peroxidation can be estimated by measurement of TBARS. TBARS can be determined by reaction of malondialdehyde a secondary breakdown product of lipid hydroperoxides, with TBA. Ethanol extracts of both Hypericum. species (HS and HT) significantly inhibit lipid peroxidation in rat brain. The inhibition of lipid peroxidation by ethanol extracts of HS and HT was the result of their scavenging effect on Fe+ 2/ascorbate generated free radicals.
Other species of Hypericum. have been shown in many experimental systems to scavenge reactive oxygen species, inhibit oxidative DNA damage and lipid peroxidation and to be effective in hypercholesterolemia (El Sherbiny et al., Citation2003; Hakimoglu et al., Citation2006; Singh et al., Citation2002; Valentao et al., Citation2002). The antioxidant potentials of ethanol extract of Hypericum perforatum. and methanol extracts of Hypericum triquetrifolium. aerial parts were also studied using different antioxidant tests, and compounds isolated from these extracts or fractions possess a significant antioxidant activity (Benedi et al., Citation2004; Conforti et al., Citation2002; Silva et al., Citation2005). In recent years, the commercially available H. perforatum.–derived products also include sophisticated phytopharmaceuticals and nutraceuticals, teas, tinctures, and juices (Gaedcke, Citation2003).
On the basis of the results obtained in the current study, we conclude that the ethanol extracts of Hypericum scabroides. and Hypericum. triquetrifolium. posesses high antioxidant activities that might be helpful in preventing or slowing the progress of various oxidative stress–related diseases. Both Hypericum. extracts studied here could also be considered as good candidates for food preservation or functional foods, as well as for pharmaceutical and natural plant–based products. Further investigations on the isolation and identification of antioxidant components in the plants may lead to chemical entities with potential for clinical use.
Acknowledgment
This work was supported by research grants from the Dicle University Research Council (DUAPK, project numbers 04-FF-55 and 03-FF-63).
References
- Ames B N, Shigenaga M K, Hagen T M. Oxidants, AOXs and the generative disease of aging. Proc Natl Acad Sci USA 1993; 90: 7915–7922
- Aruoma O I, Cuppett S L. Antioxidant Methodology In Vivo and In Vitro Concepts. AOCS Press, Champaign, IL 1997; 41–172
- Aruoma O I, Laughton M J, Halliwell B. Carnosine, homocarnosine and anserine: Could they act as antioxidants in vivo.?. Biochem J 1989; 264: 863–869
- Aurand L W, Boonme N H, Gidding G G. Superoxide and singlet oxygen in milk lipid peroxidation. J Dairy Sci. 1977; 60: 363–369
- Barnes J, Anderson L A, Phillipson J D. St. John wort (Hypericum perforatum. L.): A review of its chemistry pharmacology and clinical properties. J Pharmac Pharmacol 2001; 53: 583–600
- Baytop T. Therapy with Medicinal Plants in Turkey. Publications of Istanbul University, Istanbul 1984; 185–186
- Benedi J, Arroya R, Romero C, Martin-Aragon S, Villar A M. Antioxidant properties and protective effects of astandardized extract of Hypericum perforatum. on hydrogen peroxide-induced oxidative damage in PC12 cells. Life Sci 2004; 75: 1263–1276
- Block G, Peterson B, Subar A. Fruits, vegetables and cancer prevention: A review of the epidemiological evidence. Nutr Cancer 1992; 18: 1–29
- Borek C. Molecular mechanisms in cancer induction and prevention. Environ Health Perspect 1993; 101: 151–160
- Brand-Williams W, Cuvelier M E, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 1995; 28: 25–30
- Bridi R, Crossetti F P, Steffen V M, Henriques A T. The antioxidant activity of standardized extract of Ginkgo biloba. (EGb 761) in rats. Phytother Res 2001; 15: 449–451
- Butterweck V, Bockers T, Korte B, Wittowski W, Winterhoff H. Long-term effects of St. John's wort and hypericin on monoamine levels in rat hypothalamus and hippocampus. Brain Res 2002; 930: 21–29
- Butterweck V, Jurgenliemk G, Nahrsted A, Winterhoff H. Flavonoids from Hypericum perforatum. show antidepressant activity in the forced swimming test. Planta Med 2000; 66: 3–6
- Butterweck V, Wall A, Lieflander-Wulf U, Winterhoff H, Nahrsted A. Effects of the total extract and fractions of Hypericum perforatum. in animal assays for antidepressant activity. Pharmacopsychiatry 1997; 30: 117–124
- Byres T, Guerrero N. Epidemiologic evidence for vitamin C and vitamin E in cancer prevention. Am J Clin Nutr 1995; 62: 1385–1392
- Cakir A, Duru M E, Harmandar M, Ciriminna R, Passannanti S, Piozzi F. Comparision of the volatile oils of Hypericum Scabrum. L. and Hypericum perforatum. L. from Turkey. Flavour Frag J 1997; 12: 285–287
- Cakir A, Mavi A, Yıldırım A, Duru M E, Harmandar M, Kazaz C. Isolation and characterization of antioxidant phenolic compounds from the aerial parts of and Hypericum hyssopifolium. L. by activity-guided fractionation. J Ethnopharmacol 2003; 87: 73–83
- Chang L W, Yen W J, Huang S C, Duh P D. Antioxidant activity of sesame coat. Food Chem 2002; 78: 347–354
- Cheung L M, Cheung P CK, Ooi V EC. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem 2003; 81: 249–255
- Chung M I, Lai M H, Yen M H, Wu R R, Lin C N. Phenolics from Hypericum geminiflorum.. Phytochemistry 1997; 44: 943–947
- Conforti F, Statti A G, Tundis R, Menichini F, Houghton P. Antioxidant activity of methanolic extract of Hypericum triquetrifolium. Turra aerial part. Fitoterapia 2002; 73: 479–483
- Dasgupta N, De B. Antioxidant activity of Piper betle. L. leaf extract in vitro.. Food Chem 2004; 88: 219–224
- David J M, Barreisors A LBS, David J P. Antioxidant phenylpropanoid esters of triterpenes from Dioclea lasiophylla.. Pharm Biol 2004; 42: 36–38
- Decosterd A L, Hoffmann E, Kyburz R, Bray D, Hostettmann K. A new phloroglucinol derivative from Hypericum calycinum. with antifungal and in vitro. antimalarial activity. Planta Med 1991; 57: 548–551
- Decosterd A L, Stoeckli-Evans H, Chapuis J C, Msonthi J D, Sordat B, Hostettmann K. New hyperforin derivatives from Hypericum revolutum. Vahl with growth-inhibitory activity against a human colon carcinoma cell line. Helv Chim Acta 1989; 72: 464–471
- Dias A CP, Tomas-Barberan F A, Fernandes-Ferreria M, Ferreres F. Unusual flavonoids produced by callus of Hypericum perforatum.. Phytochemistry 1998; 48: 1165–1168
- Dinis T CP, Madeira V MC, Almeida L M. Action of phenolic derivates (acetoaminophen, salycilate and 5-aminosaly-cilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 1994; 315: 161–169
- Diplock A T. Will the ‘good fairies’ please prove to us that vitamin E lessens human degenerative disease?. Free Radic Res 1997; 27: 511–532
- El-Sherbiny D A, Khalifa A E, Attia A S, Eldenshary E ES. Hypericum perforatum. extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine. Pharmacol Biochem Behav 2003; 76: 525–533
- Emmons C L, Peterson D M, Paul G L. Antioxidant capacity of oat (Avena sativa. L.) extracts. 2. In vitro. antioxidant activity and contents of phenolic and tocol antioxidants. J Agric Food Chem 1999; 47: 4894–4898
- Farag R S, El-Baroty G S, Basuny A M. The influence of phenolic extracts obtained from the olive plant (cvs. Picual and Kronakii), on the stability of sunflower oil. Int J Food Sci Tech 2003; 38: 81–87
- Flausino O A, Zangrossi H, Salgado J V, Viana M B. Effects of acute and chronic treatment with Hypericum perforatum. L. (LI160) on different anxiety-related responses in rats. Pharmacol Biochem Behav 2002; 71: 251–257
- Gaedcke F. St. John's wort herb extracts: Manufacturing, standardisation and characterization. Hypericum, Medicinal and Aromatic Plants-Industrial Profiles, E Ernst. Taylor and Francis, London 2003; Vol. 31: 49–64
- Gao X, Bjork L, Trajkovski V, Uggla M. Evaluation of antioxidant activities of rose hips ethanol extracts in different test systems. J Sci Food Agric 2000; 80: 2021–2027
- Gordon M H. The mechanism of the antioxidant action in vitro.. Food Antioxidants, B JF Hudson. Elsevier, London 1990; 1–18
- Gülçin I, Küfrevioğlu Ö İ, Oktay M, Büyükokuroğlu M E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica. L.). J Ethnopharmacol 2004; 90: 205–215
- Gunatilaka A AL, Balasubramaniam S, Kumar V. 2,3-Dimetoxyxanthone from Hypericum mysorense.. Phytochemistry 1979; 18: 182–183
- Hakimoglu F, Kizil G, Kanay Z, Kizil M, Isi H. The effect of ethanol extract of Hypericum lysimachioides. on lipid profile in hypercholesterolemic rabbits and its in vitro. antioxidant activity. Atherosclerosis 2006; 192: 113–122
- Halliwell B, Gutteridge J MC, Auroma O I. The deoxyribose method: a simple ‘test tube’ assay for determination of rate constants for reaction of hydroxyl radicals. Anal Biochem 1987; 165: 215–219
- Hertog M GL, Feskens E JM, Hollman P CH, Katan J B, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993; 342: 1007–1011
- Ito N, Hirose M, Fukushima S, Tsuda H, Shirai T, Tatematsu M. Studies on antioxidants: Their carcinogenic and modifying effects on chemical carcinogenesis. Food Chem Toxicol 1986; 24: 1071–1082
- Kaur C, Kapoor H C. Antioxidants in fruits and vegetable-the Milleniums health. Int J Food Sci Tech 2001; 36: 727–735
- Kikuzaki H, Nakatani N. Antioxidant effects of some ginger constituents. J Food Sci 1993; 58: 1407–1410
- Kizil G, Toker Z, Özen Ç Ö, Aytekin Ç. The antimicrobial activity of essential oils of Hypericum scabrum Hypericum scabroides Hypericum triquetrifolium.. Phytother Res 2004; 18: 339–341
- Koşar M, Dorman H JD, Hiltunen R. Effect of an acid treatment on the phytochemical and antioxidant characteristics of extracts from selected Lamiaceae. species. Food Chem 2005; 91: 525–533
- Kuchandy E, Rao M NA. Oxygen radical scavenging activity of curcumin. Int J Pharmacog 1990; 58: 237–240
- Kumar A, Chattopadhyay S. DNA damage protecting activity and antioxidant potential of pudina extract. Food Chem 2007; 100: 1377–1384
- Landbo A K, Meyer A S. Ascorbic acids improves the antioxidant activity of European grape juices by improving the juices' ability to inhibit lipid peroxidation of human LDL in-vitro.. Int J Food Sci Tech 2001; 36: 727–735
- Lean L P, Mohamed S. Antioxidative and antimycotic effects of turmeric, lemon-grass, betel leaves, clove, black pepper leaves and Garcinia atriviridis. on butter cakes. J Sci Food Agric 1999; 79: 1817–1822
- Lebeau J, Furman C, Bernier J L, Duriez P, Teissier E, Cotella N. Antioxidant properties of di-tert.-butylhydroxylated flavonoids. Free Radic Biol Med 2000; 29: 900–912
- Liu F, Ng T B. Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci 2000; 66: 725–735
- Matthaus B. Antioxidant activity of extracts obtained from residues of different oilseeds. J Agr Food Chem 2002; 50: 3444–3452
- Maxwell S R, Lip G Y. Free radicals and antioxidants in cardiovascular disease. Br J Clin Pharmacol 1997; 44: 307–317
- Mitsuda H, Yasumoto K, Iwani K. Antioxidant actions of indole compounds during the autoxidation of linoleic acid. Eiyo to Shoduryou 1966; 19: 210
- Mukherjee P K, Verpoorte R, Suresh B. Evaluation of in vivo. wound healing activity of Hypericum patulum. (Family: Hypericaceae) leaf extract on different wound model in rats. J Ethnopharmacol 2000; 70: 315–321
- Ng T B, Liu F, Wang Z T. Antioxidative activity of natural products from plants. Life Sci 2000; 66: 709–723
- Osawa T, Namiki M. A novel type of antioxidant isolated from leaf wax of Eucalptus. leaves. Agric Biol Chem 1981; 45: 735–739
- Ottolenghi A. Interaction of ascorbic acid and mitocondria lipids. Arch Biochem Biophys 1959; 79: 355–363
- Oyaizu M. Studies on products of browning reaction: Antioxidative activity of products of browning reaction prepared from glucosamine. J Nutr 1986; 44: 307–315
- Ozen H Ç, Başhan M. The composition of fatty acids in Hypericum scabrum, H. scabroides H.amblysepalum.. Turk J Chem 2003; 27: 723–725
- Ozturk B, Apaydın S, Goldeli E, Ince I, Zeybek U. Hypericum triquetrifolium. Turra. extract exhibits antiinflammatory activity in the rat. J Ethnopharmacol 2002; 80: 207–209
- Pin-Der-Duh X. Antioxidant activity of burdock (Arctium lapa. linne): its scavenging effect on free radical and active oxygen. J Am Oil Chem Soc 1998; 75: 455–461
- Pistelli L, Bertolli A, Zucconelli S, Morelli I, Panizzi L, Menichini F. Antimicrobial activity of crude extracts and pure compounds of Hypericum hircinum.. Fitoterapia 2000; 71: 138–140
- Rabanal R M, Arias A, Prado B, Hernandez-Perez M, Sanchez-Mateo C C. Antimicrobial studies on three species of Hypericum. from the Canary Islands. J Ethnopharmacol 2002; 81: 287–292
- Rath G, Potterat O, Mavi S, Hostettmann K. Xanthones from Hypericum roeperanum.. Phytochemistry 1996; 43: 513–520
- Rice-Evans C A, Miller N J, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci 1997; 2: 152–159
- Richardson J S. Free radicals in the genesis of Alzheimer's disease. Ann N Y Acad Sci 1993; 695: 73–76
- Robson N KB, Strid A. Hypericum. L. Mountain Flora of Greece, Vol 1, A Strid. Cambridge University Press, Cambridge 1986; 594
- Rocha L, Marston A, Potterat O, Auxiliadora M, Kaplan C, Stoeckli-Evans H, Hostettmann K. Antibacterial phloroglucinols and flavonoids from Hypericum brasiliense.. Phytochemistry 1995; 40: 1447–1452
- Rose W M, Creighton M O, Stewart D HP, Sanval M, Trevithick G R. In vivo. effects of vitamin E on cataractogenesis in diabetic rats. Can J Ophthalmol 1982; 17: 61–66
- Saija A, Scalese M, Lanza M, Marzullo D, Bonina F, Castelli F. Flavonoids as antioxidant agents: Importance of their interaction with biomembranes. Free Radic Biol Med 1995; 19: 481–486
- Sakar M K, Tamer A U. Antimicrobial activity of different extracts from Hypericum species.. Fitoterapia 1990; 61: 464–466
- Schinel'a G R, Tournier H A, Pricto J M, Mordujovich D, Rios J L. Antioxidant activitiy of anti-inflammatory plant extracts. Life Sci 2000; 70: 1023–103
- Schwarz K, Bertelsen G, Nissen L S, Gardner P T, Heinonen M I, Hopia A, Huynh-Ba T, Lambelet P, McPhail D, Skibsted L H, Tijbur L. Investigation of plant extracts for the protection of processed foods against lipid oxidation. Comparison of antioxidant assays based on radical scavenging, lipid oxidation and analysis of the principal antioxidant compounds. Eur Food Res Technol 2001; 212: 319–328
- Schwob I, Bessiere J M, Dherbomez M, Viano J. Composition and antimicrobial activity of the essential oil of Hypericum coris.. Fitoterapia 2002; 73: 511–513
- Shimada K, Fujikava K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 1992; 40: 945–948
- Shukla S, Gupta S, Gambhir J K, Prabhu K M, Murthy P S. Antioxidant effect of aqueous extract of the bark of Ficus bengalensis. in hypercholesterolaemic rabbits. J Ethnopharmacol 2004; 92: 47–51
- Silva B A, Ferreres F, Malva J O, Dias C P. Phytochemical and antioxidant characterization of Hypericum perforatum. alcoholic extracts. Food Chem 2005; 90: 157–167
- Singh O A, Garg V, Gupta S, Kulkarni K. Role of antioxidants in chronic fatigue syndrome in mice. Indian J Exp Biol 2002; 40: 1240–1244
- Singleton V L, Orthofer R, Lamuela-Raventos R M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymolosy Oxidant and Antioxidants (Part A), Vol. 299, L Packer. Academic Press, San Diego, CA 1999; 152–178
- Sokmen A, Jones M B, Ertürk M. Antimicrobial activity of extracts from the cell cultures of some Turkish medicinal plants. Phytother Res 1999; 13: 355–357
- Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem 1997; 272: 20963–20966
- Storz P. Reactive oxygen species in tumor progression. Front Biosci 2005; 10: 1881–1896
- Sundararajan R, Haja N A, Venkatesan K, Mukherjee K, Saha B P, Bandyopadhyay A, Mukherjee P K. Cytisus scoparius link-A natural antioxidant. BMC Complement Altern Med 2006; 6: 1–7
- Tandon V, Gupta R K. Effect of Vitex negundo. on oxidative stress. Indian J Pharmacol 2005; 37: 38–40
- Tang S, Sheedan D, Buckely D C, Morrissey P A, Kerry J P. Anti-oxidant activity of addede tea catechins on lipid oxidation of raw minced red meat, poultry and fish muscle. Int J Food Sci Tech 2001; 36: 685–692
- Toker Z, Kizil G, Özen Ç Ö, Kizil M, Ertekin S. Compositions and antimicrobial activities of the essential oils of two Hypericum. species from Turkey. Fitoterapia 2006; 77: 57–60
- Trifunovic S, Vajs V, Macura S, Juranic N, Djarmati Z, Jankov R, Milosavljevic S. Oxidation products of hyperforin from Hypericum perforatum.. Phytochemistry 1998; 49: 1305–1310
- Valentao O P, Fernandes E, Carvalho F, Adrade P B, Seabre R M, Bastos M. Antioxidant activity of Hypericum androsaemum. infusion: Scavenging activity against superoxide radical, hydroxyl radical and hypochlorous acid. Biol Pharm Bull 2002; 25: 1320–1323
- Vasu V T, Modi H, Thaikoottathil J V, Gupta S. Hypolipidaemic and antioxidant effect of Enicostemma littorale. Blume aqueous extract in cholesterol fed rats. J Ethnopharmacol 2005; 101: 277–282
- Walzem R L, Watkins S, Frankel E N, Hansen R J, German J B. Older plasma lipoproteins are more susceptible to oxidation: A linking mechanism for the lipid and oxidation theories of atherosclerotic cardiovascular disease. App Biol Sci (PNAS) 1995; 92: 7460–7464
- Wu Q L, Wang S P, Du L J, Yang J S, Xiao P G. Xanthones from Hypericum japonicum H. henryi.. Phytochemistry 1998b; 49: 1395–1402
- Wu Q L, Wang S P, Du L J, Zhang S M, Yang J S, Xiao P G. Chromone glycosides and flavonoids from Hypericum japonicum.. Phytochemistry 1998a; 49: 1417–1420
- Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol 1998; 108: 101–106
- Yen G C, Duh P D. Antioxidative properties of methanolic extracts from peanut hulls. J Am Oil Chem Soc 1993; 70: 383–386