Abstract
In vitro. tumor growth inhibition by the ethanol extract of Miconia fallax. DC. (Melastomataceae) was evaluated in culture media containing HeLa cells at the following concentrations: 5, 15, 25, 35, and 45 μ g/mL. Bioassay-guided fractionation of this extract furnished a mixture of ursolic and oleanolic acids. Both the ethanol extract and the mixture of the triterpenoid acids produced dose-dependent tumor growth inhibition. This antitumor activity was more pronounced when a mixture of the triterpene acids at a concentration of 45 μ g/mL was added to a HeLa cell culture medium.
Introduction
Miconia., a genus with approximately 1000 species (Martins et al., Citation1996), belongs to the Melastomataceae family (Judd & Skean, Citation1991; Renner et al., Citation1993). Previous studies on Miconia. species have demonstrated the presence of triterpenes, coumarins, and benzoquinones in these plants (Chan et al., Citation1992; Gunatilaka et al., Citation2001; Lowry, Citation1968; Macari et al., Citation1990). In recent work undertaken in our laboratory, several crude plant extracts from Miconia. and their isolated compounds were reported to exhibit remarkable biological activities (Cunha et al., Citation2003; Resende et al., Citation2006; Spessoto et al., Citation2003; Vasconcelos et al., Citation2006). Ursolic and oleanolic acids are triterpenoid compounds that are widely distributed in the plant kingdom, and they have been frequently isolated as mutually isomeric mixtures from Miconia. species (Cunha et al. Citation2006; Vasconcelos et al., Citation2006).
Plant-derived compounds have been an important source of several clinically useful anticancer agents (Cragg & Newman, Citation2005; Pezzuto, Citation1997). In a screening for antitumor compounds obtained from plants, the antitumor activity of Miconia fallax. DC. has been investigated in this work by using culture media containing HeLa cells, a cell line of the human uterine cervix adenocarcinoma. This is the first time that this species has been investigated for antitumor activity. The bioassay-guided fractionation of ethanol extract of Miconia fallax. led to the isolation of a mixture of the triterpenes ursolic and oleanolic acids.
Materials and Methods
Plant material
M. fallax. DC. (Melastomataceae) was collected in September 2004 between the cities of Franca and Claraval, along a highway in the state of São Paulo, Brazil, and it was identified by Dr. Angela Borges Martins, Instituto de Biologia, UNICAMP, Brazil. A voucher specimen has been deposited in the herbarium of this same institute (UEC 65132).
Extraction and isolation
Dried and powdered aerial parts (0.5 kg) of M. fallax. were exhaustively extracted with ethanol. The solvent was removed under vacuum in a rotatory evaporator to yield 22.0 g of the dry crude extract. Part of this extract (13.0 g) was chromatographed on silica gel 60 (300 g, 0.063–0.200 mm; Merck, Danmstadt, Germany) by vacuum liquid chromatography, using a mixture of hexane:ethyl acetate and ethyl acetate:ethanol in increasing polarity as eluents. This procedure furnished six fractions of 1000 mL each, namely F1 (hexane:AcOEt 3:1), F2 (hexane:AcOEt 1:1), F3 (AcOEt), F4 (AcOEt:EtOH 3:1), F5 (AcOEt:EtOH 1:1), and F6 (EtOH). The active fractions 1 (1.22 g) and 2 (1.85 g) were combined. This combined fraction was purified by column chromatography on silica gel 60 (0.063–0.200 mm, Merck), affording a mixture (750 mg) containing ursolic acid (65%) and oleanolic acid (35%) (). These triterpene acids were identified by comparison with authentic samples (HPLC, 1H and 13C NMR).
In vitro. bioassay test
Cells of the human uterine cervix adenocarcinoma cell line (Pater & Pater, Citation1985), HeLa (ATCC-CCL-2), were cultured in DMEM F-12 medium (Gibco) supplemented with 10% fetal calf serum (Gibco) in a 75-cm2 cell culture flask until confluence was reached. The cells were then treated with trypsin-EDTA for detachment from the plate, centrifuged, resuspended in the same complete medium, and subcultured on 6-well plates (Nunc, Walthan, USA) containing a 22 × 22 mm glass coverslip (Corning, New York, USA), with the same cell suspension volume being added to each well. A stock solution of the samples (10 mg/mL) were prepared. The following doses were added to the medium: 5, 15, 25, 35, and 45 μ g/mL. Incubation with DMSO was also performed as negative control. The cells were then cultured for 24 h in a moist oven with 5% CO2 at 37°C. After this period, the drugs were replaced and cells were cultured once again for 24 h. After a total culture period of 48 h, the cells were fixed with 2% p.-formaldehyde in phosphate buffer (pH 7.3) for 10 min at 37°C and washed with the same buffer 3 times. The coverslips were mounted on glycerol and sealed with commercial enamel. The tumor cells were observed and documented using the Zeiss Axiophot microscope (Carl Zeiss Inc., Jena, Germany). Cells were then counted in 20 random fields on each slide with 40 × objective, and only adhered cells with adequate morphology were computed. Data were statistically analyzed.
Statistical analysis
Data were statistically analyzed by one-way ANOVA followed by Dunnett's multiple comparison test, with the level of significance set at p < 0.01 for both the ethanol extract and the mixture of ursolic acid and oleanolic acid.
Results and Discussion
The search for new therapeutic drugs for the treatment of cancer from natural products is based mainly on the cytotoxic properties of the natural samples (Goldin et al., Citation1981). In this sense, both the ethanol extract of M. fallax. and a mixture of ursolic acid and oleanolic acid isolated from this extract produced a dose-dependent tumor growth inhibition ().
Figure 2 In vitro. inhibition of tumor cell growth of the ethanol extract of Miconia fallax. and a mixture of ursolic acid and oleanolic acid. The results are shown as mean ± SD of number of HeLa cells, and a significant difference from the control group is shown as *p < 0.001 for both ethanol extract and mixture of ursolic acid and oleanolic acid.
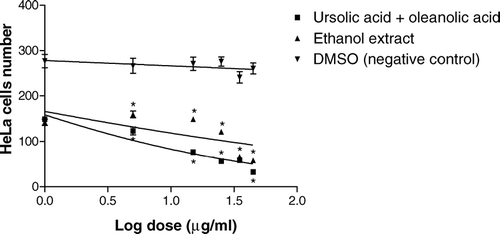
We observed that the antitumor activity is more pronounced when a mixture of the triterpene acids at a concentration of 45 μ g/mL is added to a HeLa cell culture medium. Our results show that ursolic and oleanolic acids probably are the active compounds in the crude ethanol extract of M. fallax..
Moreover, ursolic acid and oleanolic acid are of interest in the oncology field because of their cytotoxicity and antimutagenic, antiviral, and anti-invasive activities as well as their ability to decrease undesirable radiation damage to the hematopoietic tissue after radiotherapy (Hsu et al., Citation1997; Kim et al., Citation1998; Li et al., Citation2002; Liu, Citation1995). Besides that, a recent review mentions reports on the biological properties of ursolic acid and shows its main antitumor effects and chemopreventive properties in normal cells (Novotý et al., Citation2001). It has also been observed that ursolic acid is capable of inducing tumor cell apoptosis in experimental cancer cell lines (Baek et al., Citation1997; Urechk, 2005). It has been reported that treatment with ursolic acid results in concentration-dependent decreased cell viability and genomic DNA fragmentation, a hallmark of apoptosis (Urech, Citation2005).
Substantial progress has been made in medicine by using herbs or mixtures of compounds. Because these mixtures can often produce desired biological effects or even potentiate them, they have been widely employed in health care and clinical treatments (Liu, Citation2005).
Our data reinforce the importance of ursolic acid and oleanolic acid as a source of new chemotherapeutics, but additional studies are necessary to understand the mechanisms of their antitumor effects.
Acknowledgment
The authors are grateful to FAPESP (Proc. 00/03911-4 and 06/50308-8) for financial support.
References
- Baek J H, Lee Y S, Kank C M, Jung-Ae K, Kwon K S, Son H C, Kim K W. Intracellular Ca2 + release mediates ursolic acid–induced apoptosis in human leukemic HL-60 cells. Int J Cancer 1997; 73: 725–728
- Chan W R, Sheppard V, Kathleen A M, Tinto W F, Reynolds W F. Triterpenes from. Miconia stenostachya. J Nat Prod 1992; 55: 963–966
- Cragg G M, Newnman D J. Plants as source of anticancer agents. J Ethnopharmacol 2005; 100: 72–79
- Cunha W R, Crevelin E J, Arantes G M, Crotti A EM, Silva M LA, Furtado N AJC, Albuquerque S, Ferreira D S. A study of the trypanocidal activity of triterpene acids isolated from Miconia. species. Phytother Res 2006; 20: 474–478
- Cunha W R, Martins C, Ferreira D S, Crotti A EM, Lopes N P, Albuquerque S. In vitro. trypanocidal activity of triterpenes from Miconia species. Planta Med 2003; 69: 470–472
- Goldin A, Venditt J M, Macdonald J S, Muggia F M, Henney J E, Devita V T. Currents results of the screening program at the division of cancer treatment, National Cancer Institute. Eur J Cancer 1981; 17: 129–142
- Gunatilaka A AL, Berger J M, Evans R, Miller J S, Wisse J H, Neddermann K M, Bursuker I, Kingston D GI. Isolation, synthesis and structure-activity relationships of bioative benzoquinones from Miconia lepidota from the Suriname rainforest. J. Nat Prod 2001; 64: 2–5
- Hsu H Y, Yang J J, Lin C C. Effects of oleanoic acid and ursolic acid on inhibiting tumor growth and enhancing the recovery of heamatopoietic system postirradiation in mice. Cancer Lett 1997; 111: 7–13
- Judd W S, Skean J D, Jr. Taxonomic studies in Miconieae. (Melastomataceae). Bull Florida Mus Nat Hist 1991; 36: 25–84
- Kim S H, Ahn B-Z, Ryu S Y. Antitumor effects of ursolic acid isolated from. Oldenlandia diffuse. Phytother Res 1998; 12: 553–556
- Li J, Guo W J, Yang Q Y. Effects of ursolic acid and oleanoic acid on human colon carcinoma cell line HCT15. World J Gastroenterol 2002; 8: 493–495
- Liu J. Pharmacology of oleanoic acid and ursolic acid. J Ethnopharmacol 1995; 49: 57–68
- Liu J. Oleanoic acid and ursolic acid: Research perspectives. J Ethnopharmacol 2005; 100: 92–94
- Lowry J B. The distribuition and potential taxonomic value of alkylated ellagic acids. Phytochemistry 1968; 7: 1803–1813
- Macari P AT, Emerenciano V P, Ferreira Z MGS. Identificação dos triterpenos de Miconia albicans. Triana através de análise por microcomputador. Quím Nova 1990; 13: 260–262
- Martins A B, Semir J, Goldenberg R, Martins E. O gênero Miconia. Ruiz & Pav. (Melastomataceae) no estado de São Paulo. Acta Bot Bras 1996; 10: 267–316
- Novotý L, Vachálková A, Biggs D. Ursolic acid: An anti-tumorigenic and chemopreventive activity. Neoplasma 2001; 48: 241–246
- Pater M M, Pater A. Human papillomavirus types 16 and 18 sequences in carcinoma cell lines of the cervix. Virology 1985; 145: 313–318
- Pezzuto J M. Plant-derived anticancer agents. Biochem Pharmacol 1997; 53: 121–133
- Renner S S. Phylogeny and classification of the Melastomataceae and Memecylaceae. Nord J Bot 1993; 13: 519–540
- Resende F A, Barcala C AMA, Faria M CS, Kato F H, Cunha W R, Tavares D C. Antimutagenicity of ursolic acid and oleanolic acid against doxorubicin-induced clastogenesis in Balb/c mice. Life Sci 2006; 79: 1268–1273
- Spessoto M A, Ferreira D S, Crotti A EM, Silva M LA, Cunha W R. Evaluation of the analgesic activity of extracts of Miconia rubiginosa (Melastomataceae). Phytomedicine 2003; 10: 606–609
- Urech K, Scher J M, Hostanska K, Becker H. Apoptosis inducing activity if viscin, a lipophilic extract from Viscum album. L. J Pharm Pharmacol 2005; 57: 101–109
- Vasconcelos M A, Royo V A, Ferreira D S, Crotti A EM, Silva M LA, Carvalho J CT, Bastos J K, Cunha W R. In vivo. analgesic and anti-inflammatory activities of ursolic acid and oleanolic acid from Miconia albicans. (Melastomataceae). Z Naturforsh C 2006; 61: 477–482