Abstract
Rumex ecklonianus. Meissner (Polygonaceae) is a wild South African herb whose leaves are edible when young. It is mildly purgative and is used in the treatment of chlorosis and anemia. The polyphenolic content and antioxidant and antibacterial activity of the acetone, methanol, and water extracts were determined in this study. The concentrations of the different classes of phenolic compounds were higher in the acetone and methanol extracts when compared with the water extracts; this also correlates highly with the total phenolic content. Antioxidant activities of acetone and methanol extracts as assessed by three established in vitro. methods, namely, 2,2-azinobis.-(3-ethyl benzothiazoline-6-sulfonic acid) (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH), and ferric reducing antioxidant power (FRAP) methods were comparable with that of butylated hydroxyl toluene. The extracts showed significant activity against both Gram-positive and Gram-negative bacteria. The strongest antibacterial activity was found in the acetone extract, whose activity was pronounced on 7 of the 10 bacterial strains used, with a MIC value of 2.0 mg/mL. None of the extracts, however, inhibited Staphylococcus epidermidis. or Salmonella pooni.. The data obtained in this study suggest that R. ecklonianus. may be a good candidate for functional foods as well as pharmaceutical plant-based products. This is the first report on the biological activity of R. ecklonianus..
Introduction
There is a great deal of evidence indicating that excessive free radical production and lipid peroxidation are actively involved in the pathogenesis of more than 100 diseases including malaria, atherosclerosis, cancer, diabetes, acquired immunodeficiency syndrome, and heart diseases (Luximon-Ramma et al., Citation2002; Yildrim et al., Citation2000). Plant-derived antioxidants such as ascorbic acid, α -tocopherol, polyphenols such as phenolics acids, flavonoids, proanthocyanidins, among others, are becoming increasingly suggested as important dietary factors (Cantuti-Castelvetri et al., 2000; Ferguson, Citation2001).
Phenolic compounds are plant secondary metabolites, which play an important role in disease-resistance protection against pests and species dissemination. The interest in these compounds is related with their antioxidant activity and promotion of health benefits (Balasundram, Citation2006). The growing interest in the substitution of synthetic antioxidants with natural ones has fostered research on plant sources and the screening of raw materials for identifying new ones. Furthermore, it has been suggested that there is an inverse relationship between dietary intake of antioxidant-rich foods and the incidence of human diseases (Lu & Foo, Citation2000; Yildrim et al., 2001).
Rumex ecklonianus. Meissner (Polygonaceae) belong; to a genus of about 200 species. It is native to South Africa and is commonly known as South African Dock. This species is a perennial plant that grows abundantly in the wild. The fleshy to leathery leaves form a basal rosette at the root, have minor leaf veins, and the leaf blade margins are entire or crenate (Anonymous, Citation2004). The inconspicuous flowers are carried above the leaves in whorl-like clusters with the fertile flowers, mostly hermaphrodite. The flowers and seeds grow on long clusters at the top of a stalk emerging from the basal rosette. It has been documented that the leaves of this species are edible when young and are occasionally used like spinach (Bhat & Rubuluza, Citation2002). Chrysophanic acid and emodin are chemical compounds that have been isolated from this species. It is mildly purgative and is used in the treatment of chlorosis and anemia (Anonymous, Citation2007).
In recent years, multiple-drug resistance in human pathogenic microorganisms has developed, due to indiscriminate use of commercial antimicrobial drugs commonly used in the treatment of infections. This situation has forced scientists to search for new antimicrobial substances from various plants (Karaman et al., Citation2003). As far as our literature survey could ascertain, there is no information about the antioxidant and antibacterial activity of this plant. Therefore, the aim of the current work was to evaluate the antioxidant and antibacterial potential of acetone, methanol, and water extracts of Rumex ecklonianus., as well as its phenolic content.
Materials and Methods
Collection of plant material and preparation of extracts
Fresh plant material of Rumex ecklonianus. was collected in November 2006 from the wild around the University of Fort Hare campus (Alice, South Africa). Professor D. Grierson of the Department of Botany, University of Fort Hare, authenticated the species. A voucher specimen was prepared and deposited in the herbarium of the Department of Botany (Jimoh Med. 2006/7). The plant material was allowed to air-dry at ambient temperature (± 24°C) and then milled. Twenty grams each of the sample were extracted with 200 mL each of acetone, methanol, and water, respectively, at ambient temperature, with agitation for 18–24 h. Each extract was filtered using Whatman no. 1 filter paper and concentrated under reduced pressure to dryness below 40°C. The water extract was freeze-dried. The extract yields (w/w) were acetone (1.70%), methanol (5.34%), and water (7.85%), respectively. The dried extracts thus obtained were used directly for the determination of the antioxidant and antibacterial activities.
Chemicals used for experimentation
1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2,2′ -azinobis.-3ethylbenzothiazoline-6-sulfonic acid (ABTS), 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4′-disulfonic acid, potassium ferricyanide, catechin, butylated hydroxytoluene (BHT), ascorbic acid, catechin, tannic acid, quercetin, and FeCl3 were purchased from Sigma Chemical Co. (St. Louis, MO, USA); vanillin from BDH Chemical Ltd. (Poole, Dorsetshire, UK); Folin-Ciocalteu phenol reagent and sodium carbonate were from Merck Chemical Supplies (Damstadt, Germany). All the other chemicals used, including the solvents, were of analytical grade.
Determination of total phenolics
Total phenol contents in the extracts were determined by the modified Folin-Ciocalteu method (Wolfe et al., Citation2003). An aliquot of the extract was mixed with 5 mL Folin-Ciocalteu reagent (previously diluted with water 1:10, v/v) and 4 mL (75 g/L) of sodium carbonate. The tubes were vortexed for 15 s and allowed to stand for 30 min at 40°C for color development. Absorbance was then measured at 765 nm using the Hewlett Packard UV-Vis spectrophotometer. Samples of extract were evaluated at a final concentration of 1.0 mg/mL. Total phenolic content was expressed as mg/g tannic acid equivalent using the following equation based on the calibration curve: y. = 0.1216x., R2 = 0.9365, where x. was the absorbance and y. was the tannic acid equivalent (mg/g).
Determination of total flavonoids
Total flavonoids were estimated using the method of Ordon ez et al. (Citation2006). To 0.5 mL of sample, 0.5 mL of 2% AlCl3 ethanol solution was added. After 60 min at room temperature, the absorbance was measured at 420 nm. A yellow color indicated the presence of flavonoids. Extract samples were evaluated at a final concentration of 0.1 mg/mL. Total flavonoid contents were calculated as quercetin (mg/g) using the following equation based on the calibration curve: y. = 0.0255x., R2 = 0.9812, where x. was the absorbance and y. was the quercetin equivalent (mg/g).
Determination of total flavonols
Total flavonols were estimated using the method of Kumaran and Karunakaran (Citation2007). To 2.0 mL of sample (standard), 2.0 mL of 2% AlCl3 ethanol and 3.0 mL (50 g/L) sodium acetate solutions were added. The absorption at 440 nm was read after 2.5 h at 20°C. Extract samples were evaluated at a final concentration of 0.1 mg/mL. Total flavonoid contents were calculated as quercetin (mg/g) using the following equation based on the calibration curve: y. = 0.0255x., R2 = 0.9812, where x. was the absorbance and y. was the quercetin equivalent (mg/g).
Determination of total proanthocyanidins
Determination of proanthocyanidin was based on the procedure reported by Sun et al. (Citation1998). A volume of 0.5 mL of 0.1 mg/mL of extract solution was mixed with 3 mL of 4% vanillin-methanol solution and 1.5 mL hydrochloric acid; the mixture was allowed to stand for 15 min. The absorbance was then measured at 500 nm. Total proanthocyanidin contents were expressed as catechin equivalents (mg/g) using the following equation based on the calibration curve: y. = 0.5825x., R2 = 0.9277, where x. was the absorbance and y. was the catechin equivalent (mg/g).
Determination of antioxidant activity
ABTS radical scavenging assay
For the ABTS assay, the method of Re et al. (Citation1999) was adopted. The stock solutions included 7 mM ABTS. + solution and 2.4 mM potassium persulfate solution. The working solution was then prepared by mixing the two stock solutions in equal quantities and allowing them to react for 12 h at room temperature in the dark. The solution was then diluted by mixing 1 mL ABTS. + solution with 60 mL of methanol to obtain an absorbance of 0.706 ± 0.001 units at 734 nm. Fresh ABTS. + solution was prepared for each assay. Plant extracts (1 mL) were allowed to react with 1 mL of the ABTS. + solution, and the absorbance was taken at 734 nm after 7 min using a spectrophotometer. The ABTS. + scavenging capacity of the extract was compared with that of BHT and percentage inhibition calculated as ABTS radical scavenging activity (%) = [(Abscontrol−Abssample)]/(Abscontrol)] × 100, where Abscontrol was the absorbance of ABTS radical + methanol; Abssample was the absorbance of ABTS radical + sample extract/standard.
DPPH radical scavenging assay
The effect of extracts on DPPH radical was estimated using the method of Liyana-Pathirana and Shahidi (Citation2005). A solution of 0.135 mM DPPH in methanol was prepared, and 1.0 mL of this solution was mixed with 1.0 mL of extract in methanol containing 0.02–0.1 mg of the extract. The reaction mixture was vortexed thoroughly and left in the dark at room temperature for 30 min. The absorbance of the mixture was measured spectrophotometrically at 517 nm. Ascorbic acid and BHT were used as references. The ability to scavenge DPPH radical was calculated by the following equation: DPPH radical scavenging activity (%) = [(Abscontrol−Abssample)]/(Abscontrol)] × 100, where Abscontrol was the absorbance of DPPH radical + methanol; Abssample was the absorbance of DPPH radical + sample extract /standard.
Total antioxidant activity (FRAP assay)
A modified method of Benzie and Strain (Citation1996) was adopted for the FRAP assay. The stock solutions included 300 mM acetate buffer (3.1 g C2H3NaO2· 3H2O and 16 mL C2H4O2), pH 3.6, 10 mM TPTZ (2,4,6-tripyridyl-S.-triazine) solution in 40 mM HCl, and 20 mM FeCl3· 6H2O solution. The fresh working solution was prepared by mixing 25 mL acetate buffer, 2.5 mL TPTZ, and 2.5 mL FeCl3· 6H2O. The temperature of the solution was raised to 37°C before use. Plant extracts (150 μ L) were allowed to react with 2.85 mL of the FRAP solution for 30 min in the dark. Readings of the colored product (ferrous tripyridyltriazine complex) were taken at 593 nm. The standard curve was linear between 200 and 1000 μ M FeSO4. Results were expressed in μ M Fe (II)/g dry mass and compared with that of BHT, ascorbic acid, and catechin.
Antibacterial assay
Laboratory isolates of 10 bacteria species, which included five Gram-positive and five Gram-negative strains, were obtained from the Department of Microbiology, Rhodes University. They were Bacillus cereus., Staphylococcus epidermidis., Staphylococcus aureus., Micrococcus kristinae., Streptococcus pyogenes., Escherichia coli., Salmonella pooni., Serratia marcescens., Pseudomonas aeruginosa., and Klebsiella pneumoniae.. Each organism was maintained on nutrient agar slants and was recovered by subculturing in nutrient broth (Biolab No. 2) for 24 h. Before use, each bacterial culture was diluted 1:100 with fresh sterile nutrient broth.
Adopting the method of Meyer and Afolayan (Citation1995), the bacteria were streaked in radial patterns on the agar plates and incubated at 37°C and examined after 24 and 48 h. Complete suppression of growth by a specific concentration of an extract was required to be declared active. Each extract was tested at 5.0, 2.0, 1.0, 0.5, and 0.1 mg mL− 1. Blank plates containing only nutrient agar, and other sets containing nutrient and 2% acetone/methanol/water served as controls. Acetone has been reported to be nontoxic to the organisms at 2% (Meyer & Afolayan, Citation1995).
Statistical analysis
The experimental results were expressed as mean ± standard deviation (SD) of three replicates. Where applicable, the data were subjected to one-way analysis of variance (ANOVA), and differences between samples were determined by Duncan's multiple range test using the Statistical Analysis System (SAS, 1999) program. p values < 0.05 were regarded as significant and p values < 0.01 as very significant. Correlation between polyphenol contents and antioxidant activity was established by regression analysis.
Results and Discussion
Polyphenolic contents
Phenolic compounds are very important plant constituents because they exhibit antioxidant activity by inactivating lipid free radicals or preventing decomposition of hydroperoxides into free radicals (Pokorny, Citation2001). The total phenol, flavonoids, flavonol, and proanthocyanidin content of the different extracts were determined in this study (). The concentration of phenolic compounds was higher in the acetone and methanol extracts when compared with the water extract. Differences in polarity (and thus different extractability) of the antioxidative components are obviously the reasons why phenolic compounds and antioxidant activity of the extracts differ. Similar observations were reported by Su et al. (Citation2007). The correlation between total phenol, total flavonoids, total flavonol, and total proanthocyanidins were 0.91, 0.99, and 0.99, respectively. This implies that extracts with the highest concentration of total phenols also had higher concentrations of the different classes of phenolic compounds examined.
Table 1 Polyphenol contents and antioxidant activity of the different extracts of R. ecklonianus..
DPPH radical scavenging activity
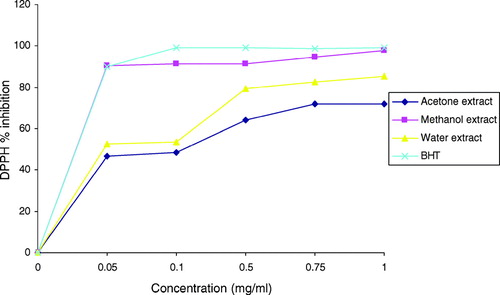
Percent DPPH scavenging activities of all the extracts were concentration dependent (); like the amount of phenolic compounds, the highest percent DPPH scavenging activity was shown by the methanol extract (97.7%) of this species, and the second highest activity was observed in the water extract (85.3%), whereas acetone extract recorded the least (72.1%). It was also observed that the scavenging activity of the methanol extract was comparable with that of BHT (99.3%) at 1.0 mg/mL, which was the highest concentration tested. Correlations between the amount of phenolic compounds and percent DPPH scavenging activity of the extract was low (r = 0.363), but correlation studies on the contribution of the different classes of these phenolics compounds to DPPH scavenging activity showed that the proanthocyanidins were more involved with R value of 0.41, whereas flavonoids and flavonols were only 0.11 and 0.29, respectively. The pronounced antioxidant properties of proanthocyanidins have been reported (Luximon-Ramma et al., Citation2002). Indeed, it is worth mentioning that the common notion that antiradical activity strictly correlates with total polyphenol concentration does not always hold true (Chinnici et al., Citation2004). Similar finding of low correlations between DPPH radical scavenging activity and phenolic compounds have also been reported (Agbor et al., Citation2005; Maisuthisakul et al., Citation2007).
ABTS radical scavenging activity
It is well established that the radical system used for antioxidant evaluation may influence the experimental results, and two or more radical systems are required to investigate the radical scavenging capacities of a selected antioxidant (Yu et al., Citation2002). To better examine their antioxidant capacities, the samples extracted were also analyzed for free radical scavenging activity against ABTS.
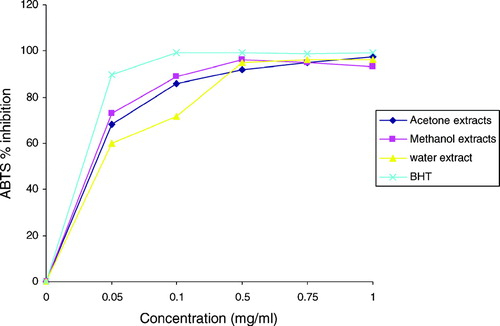
Acetone, methanol, and water extracts of R. ecklonianus. were evaluated and compared for their ABTS scavenging capacities with BHT at the same concentrations. All the tested botanical extracts show significant ABTS scavenging capacity (), which was comparable with that of BHT. Higher concentrations of the extracts were observed to be more effective in quenching free radicals in the system. The moderate correlation between ABTS radical scavenging activity of the extract and total phenolic content (r = 0.5777) implies that it is not only phenolic compounds that contributed to the radical scavenging action. This same observation was made in the DPPH assay. Because radical scavenging involves donation of a hydrogen atom or electron, there might be some other chemical compounds responsible for this action other than phenolics in this species. This will be interesting for further studies.
Total antioxidant power (FRAP)
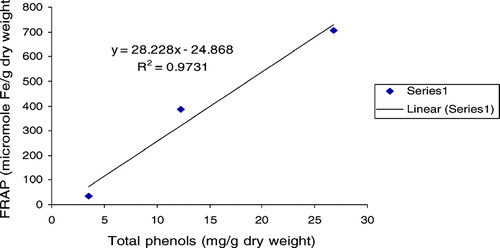
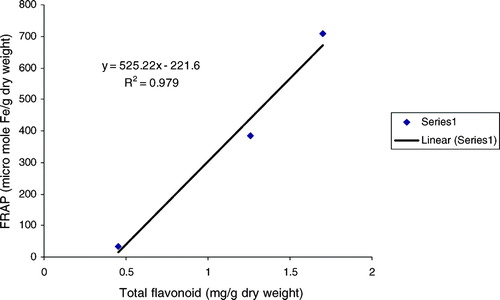
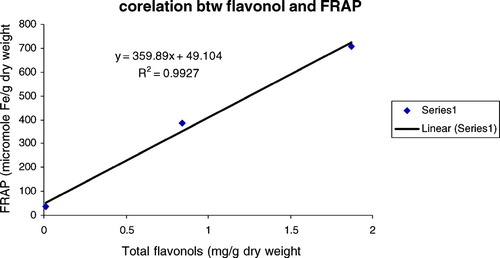
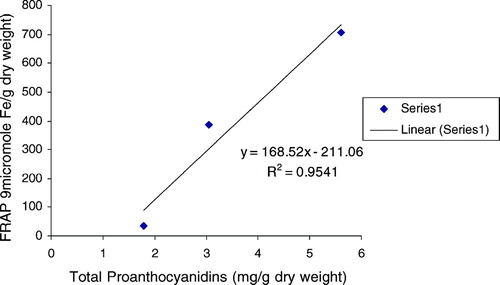
The ferric reducing/antioxidant power (FRAP assay) is widely used in the evaluation of the antioxidant component in dietary polyphenols (Luximon-Ramma et al., Citation2002). As also displayed in , the FRAP values for the investigated extract varied in a wide range between 34.30 and 707.26. Antioxidant activity increase proportionately to the polyphenol content: a linear relationship between FRAP values and total polyphenol, flavonoids, flavonols, and proanthocyanidins were established (, , , ), depicting the influence of these classes of phenolic compounds on the antioxidant capacity as expressed by the ferric reducing antioxidant power. A very high correlation coefficient was observed between FRAP and all the classes of phenolic compounds. The methanol extract being relatively high in all polyphenolic classes investigated expressed the most significant antioxidant activity among the various extracts. The results strongly suggest that although some other compounds could be involved in radical scavenging activity of this plant, polyphenol content is directly related to its overall antioxidant ability.
Antibacterial activity
The results of the antibacterial assay of the different extracts of R. ecklonianus. evaluated are presented in . With the exception of Staphylococcus epidermidis. and Salmonella pooni., either acetone or methanol extracts demonstrated activity against both Gram-positive and Gram-negative bacteria tested in this study; the highest MIC was 5.0 mg/mL. However, antibacterial activity of the water extract was only detected against Micrococcus kristinae, Escherichia coli., and Serratia marcescens..
Table 2 Antibacterial activity of the different extracts of R. ecklonianus..
The activity of this plant extracts against the Gram-negative bacteria is quite noteworthy as high resistance of this group of bacteria has been earlier reported (Afolayan, Citation2003; Nikaidu & Vaara, 1985). Streptococcus pyrogenes., Pseudomonas aeruginosa., and Klebsiella pneumoniae. are known pathogens of respiratory infections (Rojas, Citation2001); their inhibition by the extracts might suggest their possible use in the treatment of chest and respiratory infections.
Furthermore, Escherichia coli., which is a Gram-negative bacterium, was also inhibited by all the extracts. Although it belongs to the normal flora of humans, an enterohemorrhagic strain of E. coli. has caused serious food poisoning, and preservatives to eliminate its growth are needed (Buchanan & Doyle, Citation1997; Gulcin et al., Citation2003); the extracts of R. ecklonianus. might therefore be of use. In this study, the antibacterial properties of R. ecklonianus. were not as effective as the commercial drugs, but microorganisms become resistant to antibiotics over time. Because major attention has recently been devoted to natural sources of antioxidant and antibacterial materials, the data obtained in this study suggest a possible use of R. ecklonianus. as a source of natural antioxidant and antimicrobial agents.
Acknowledgment
The National Research Foundation of South Africa is acknowledged for financial support.
References
- Afolayan A J. Extracts from the shoots of Arctotis arctotoides. inhibit the growth of bacteria and fungi. Pharm Biol 2003; 41: 22–25
- Agbor G A, Oben J E, Ngogang J Y, Xinxing C, Vinson J A. Antioxidant capacity of some herbs/spices from Cameroon: A comparative study of two methods. J Agric Food Chem 2005; 53: 6819–6824
- Anonymous. Rumex. 2004, Available at http://www.reference.com/browse/wiki/Rumex
- Anonymous. Rumex. 2007, Available at http://www.reference.com/browse/wiki/Rumex
- Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem 2006; 99: 191–203
- Benzie I FF, Strain J J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem 1996; 239: 70–76
- Bhat R B, Rubuluza T. The biodiversity of traditional vegetables of the Transkei region in the Eastern Cape of South Africa. S Afr J Bot 2002; 68(1)94–99
- Buchanan R L, Doyle M P. Food borne disease significance of Escherichia coli. 0157:H7 and other enterohemorrhagic E. coli.. Food Technol 1997; 51(10)69–76
- Cantuti-Canstelvetri I, Shukitt-Hale B, Joseph J A. Neurobehavioural aspects of antioxidants in aging. Int J Dev Neurosci 2000; 18: 367–381
- Chinnici F, Bendini A, Gaiani A, Riponi C. Radical scavenging activities of peels and pulps from cv. Golden Delicious apples as related to their phenolic composition. J Agr Food Chem 2004; 52: 4684–4689
- Ferguson L R. Role of plant polyphenols in genomic stability. Mutat Res 2001; 475: 89–111
- Gulcin I, Oktay M, Kirecci E, Kufrevioglu I O. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum. L.) seed extracts. Food Chem 2003; 83: 371–382
- Karaman Y, Sahin F, Gulluce M, Ogutcu H, Sengul M, Adiguzel A. Antimicrobial activity of aqueous and methanol extracts of Juniperus oxycedrus. L. J Ethnopharmacol 2003; 85: 213–235
- Kumaran A, Karunakaran R J. In vitro antioxidant activities of methanol extracts of Phyllanthus. species from India. Lebens-Wiss Technol 2007; 40: 344–352
- Liyana-Pathiranan C M, Shahidi F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum. L) as affected by gastric pH conditions. J Agr Food Chem 2005; 53: 2433–2440
- Lu Y, Foo Y. Antioxidant radical scavenging activities of polyphenols from apple pomace. Food Chem 2000; 68: 81–85
- Luximon-Ramma A, Bahorun T, Soobrattee A M, Aruoma O I. Antioxidant activities of phenolic, proanthocyanidin and flavonoid components in extracts of Acacia fistula.. J Agr Food Chem 2002; 50: 5042–5047
- Maisuthisakul P, Suttajit M, Pongsawatmanit R. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem 2007; 100: 1409–1418
- Meyer J JM, Afolayan A J. Antibacterial activity of Herlichrysum aureonitens. (Asteraceae). J Ethnopharmacol 1995; 47: 109–111
- Ordon ez A AL, Gomez J D, Vattuone M A, Isla M I. Antioxidant activities of Sechium edule. (Jacq.) Swart extracts. Food Chem 2006; 97: 452–458
- Pokorny J. Introduction. Antioxidant in Foods: Practical Applications, J Pokorny, N Yanishlieva, N H Gordon. Woodhead Publishing Limited, Cambridge 2001; 1–3
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26: 1231–1237
- Rojas G, Levaro J, Tortoriello J, Navarro V. Antimicrobial evaluation of certain plants used in Mexican traditional medicine for the treatment of respiratory diseases. J Ethnopharmacol 2001; 74: 97–101
- Su L, Yin J, Charles D, Zhou K, Moore J, Yu L. Total phenolic contents, chelating capacities, and radical-scavenging properties of black peppercorn, nutmeg, rosehip, cinnamon, and oregan leaf. Food Chem 2007; 100: 990–997
- Sun J S, Tsuang Y W, Chen I J, Huang W C, Hang Y S, Lu F J. An ultra-weak chemiluminescence study on oxidative stress in rabbits following acute thermal injury. Burns 1998; 24: 225–231
- Wolfe K, Wu X, Liu R H. Antioxidant activity of apple peels. J Agr Food Chem 2003; 51: 609–614
- Yıldırım A, Mavi A, Kara A A. Determination of antioxidant and antimicrobial activities of Rumex crispus. L. extracts. J Agr Food Chem 2001; 49: 4083–4089
- Yildrim A, Mavi A, Oktay M, Kara A A, Algur O F, Bilaloglu V. Comparison of antioxidant and antimicrobial activities of tilia (Tilai arenta. Desf ex DC), sage (Salvia triloba. L.) and black tea (Camellia sinensis.) extracts. J Agr Food Chem 2000; 48: 5030–5034
- Yu L, Haley S, Perret J, Harris M, Wilson J, Qian M. Free radical scavenging properties of wheat extracts. J Agr Food Chem 2002; 50: 1619–1624