Abstract
Dichloromethane, ethyl acetate, methanol, and aqueous extracts obtained from 16 Spanish medicinal plants were screened for their antioxidant and antifungal activities. The radical scavenging capacity was evaluated by the DPPH method using a rapid screening by TLC and a spectrophotometric assay. Polar extracts obtained from Jasonia glutinosa L. (Lamiaceae), Tanacetum parthenium (L.) Schultz (Lamiaceae), Equisetum telmateia Ehrh. (Equisetaceae), Verbena officinalis L. (Verbenaceae), and Lythrum salicaria L. (Lythraceae) showed high antioxidant properties. Among them, the methanol extract of Lythrum salicaria showed the strongest antiradical capacity with an IC50 value similar to the positive control ascorbic acid. On the contrary, the best antifungal properties against Rhizopus stolonifer were produced by ethyl acetate or dichloromethane extracts from Anthemis arvensis L. subsp. arvensis (Asteraceae), Tanacetum parthenium, Santolina chamaecyparissus L. subsp. squarrosa Nyman (Asteraceae), Anagallis arvensis L. (Primulaceae) and the methanol extract of Anagallis foemina Miller (Primulaceae). The dichloromethane extract of Anthemis arvensis subsp. arvensis was the best inhibitor of fungus growth.
Introduction
Deterioration of food quality occurs during processing and storage, and it is related to oxidative processes and microorganisms. Oxidative degradation affects lipids mainly, but also carbohydrates, proteins, and nucleic acids. Many additives have long been used by the food industry to preserve their products from oxidation, usually synthetic antioxidants such as butylhydroxyanisole (BHA) and butylhydroxytoluene (BHT), with no clear effects after chronic consumption (CitationImaida et al. 1983; CitationYanishlieva, 2001). Many plants are a source of compounds with antioxidant activity that might be used as natural preservatives (CitationDorman et al., 2003; CitationCai et al., 2004; CitationAnsari et al., 2005; CitationChen et al., 2005; CitationHinneburg et al., 2006; CitationHuang et al., 2006; CitationSpeisky et al., 2006; CitationWong & Kitts, 2006). In addition, antioxidants play a special role in human health care as in past years, prevention of cardiovascular diseases and cancer has been associated with the intake of fruits and veges rich in natural antioxidants (CitationPatterson et al., 1990; CitationPietta, 2000; CitationSun, 1990 CitationHertog et al., 1993). Reactive oxygen species (ROS) are a class of highly reactive molecules derived from oxygen and generated by metabolic processes in human beings and by some external factors such as pollution, radiation, or some dietary habits. ROS can cause DNA mutation, protein oxidation, and lipid peroxidation (CitationCascales, 1999), contributing to the development of atherosclerosis (CitationSteinbrecher et al., 1990; CitationRoss, 1993; CitationNavab et al., 1995; CitationFernando et al., 1998), inflammation (CitationMaron, 2004), neurodegenerative diseases (CitationCascales, 1999; CitationFinkel & Holbrook, 2000; CitationFrank & Gupta, 2005), cataracts (CitationMartensson et al., 1989; CitationHankinson et al., 1992), cancer (CitationSun, 1990; CitationIngram et al., 1997; CitationCascales, 1999; CitationFinkel & Holbrook, 2000; CitationYanishlieva, 2001), and aging (CitationCascales, 1999; CitationFinkel & Holbrook, 2000).
On the other hand, fruits and vegetables suffer from infections during harvesting and packing. Rhizopus stolonifer is reported to cause decay in stone fruits, particularly peaches, but also in strawberries, raspberries, and grapes (CitationNorthover & Zhou, 2002; CitationKarabulut et al., 2004; CitationMari et al., 2004). The disease normally develops after harvest, during transportation, and as the fruit ripens prior to consumption. The postharvest losses from rhizopus rot are greatly increased by the rapid spread of the fungus to adjacent fruits during ripening because the pathogen is not efficiently controlled by registered fungicides and treatments (CitationKarabulut et al., 2004). In the attempt to reduce the use of chemicals because of concern about human health and environmental pollution, new alternative control approaches are being developed such as the use of medicinal and aromatic plant extracts. Plant extracts contain secondary metabolites, some of them with antimicrobial properties. The antifungal activity of essential oils (CitationCafarchia et al., 2002; CitationMeepagala et al., 2002; CitationPortillo-Ruiz et al., 2005; CitationCavaleiro et al., 2006; CitationPyun & Shin, 2006), saponins (CitationDe Lucca et al., 2006), and some alkaloids (CitationBahceevli et al., 2005) has been reported.
The aim of this work was to analyze the antioxidant and antifungal activities of medicinal plant extracts by in vitro approaches. The antioxidant capacity was measured by the DPPH method (CitationBlois, 1958; CitationBrand-Williams et al., 1995; CitationBondet et al., 1997) and the antifungal activity by in vitro cell culture of Rhizopus stolonifer. In this work 16 species traditionally used in Spain by the rural population are studied. Most of them belong to the Asteraceae family and were collected in the region of Navarra, situated in the north of the Iberian Peninsula.
Materials and Methods
Chemicals
2,2-Diphenyl-1-picrylhydrazyl (DPPH) and diphenylboric acid-2-amino ethyl ester were purchased from Sigma (St Louismo, USA.). Ascorbic acid and 2,6-di-tert-butyl-4-hydroxytoluene (BHT) was from Fluka (Buchs, SG, Switzerland). Vanillin was obtained from Merck (Darmstadt, Germany). All other chemicals and solvents were of analytical grade and purchased from common sources.
Plant material
The plants were collected in Navarra (Spain) between May and October 2004 and were identified by Dr. Rita Yolanda Cavero and Silvia Akerreta (Department of Plant Biology, University of Navarra). Voucher specimens have been deposited in the PAMP Herbarium of the University of Navarra. All species are listed in .
Table 1 Medicinal plants from Spain and traditional uses.
Herbal extracts
Plant material was air-dried in the dark at room temperature. The dried-powder (180 mesh) was successively extracted 3-times with 200 mL dichloromethane, ethyl acetate, methanol, and water after maceration at 4°C for 24 h. The dichloromethane, ethyl acetate, and methanol extracts were dried under reduced pressure at 30°C in a rotary evaporator (Büchi R-114, Flawil, Switzerland) and the aqueous extracts were lyophilized (Virtis BT3-SL, New York, USA). The dry extracts were stored in glass vials at −40°C until tested and analyzed.
DPPH free radical scavenging capacity by TLC
In order to detect antioxidant activity, a method based on the reduction of DPPH was carried out (CitationBlois, 1958). DPPH is a stable, purple colored free radical, that turns to yellow in presence of free radical scavengers. In this study, 200 μ g of crude extracts were spotted on silica-gel 60 F254 plates (Merck) and were developed in adequate solvent systems: petroleum ether:EtOAc (1:1) (dichloromethane extracts); EtOAc:MeOH:H2O (65:15:5) (ethyl acetate and methanol extracts); EtOAc:MeOH:H2O (60:20:5) (aqueous extracts). Three plates were prepared under the same conditions, one for the antiradical test and the others detected with plant drug reagents (CitationWagner & Bladt, 1996) with the aim to establish a relation between the antioxidant activity and the nature of the active compounds. The first plate was sprayed with a methanol solution of DPPH (2 mg/mL). Antiradical activity is observed as yellow spots on purple background. Ascorbic acid and BHT were used as positive controls. The second plate was detected with vanillin/H2SO4 reagent, heated at 110°C for 5 min, and observed to detect different groups of compounds (orange-yellow spots indicate polyphenolic compounds). The third silica gel plate was sprayed with Natural Products–PEG reagent and observed at UV 365 nm (flavonoids are detected as yellow-orange fluorescent spots).
DPPH photometric assay
The capacity of the plant extracts to scavenge DPPH free radicals was measured by a spectrophotometric method (CitationBrand-Williams et al., 1995) modified as follows. Dry extracts were resuspended in proper solvents. A solution of the extracts (150 μ L), at different concentrations (2000–2 μ g/mL), was mixed with 150 μ L of a DPPH methanol solution (0.04 mg/mL). The absorbance was measured at 517 nm after 30 min of reaction at room temperature in an iEMS Reader Labsystems (Helsinki, Finland). Controls contained all the reaction reagents except the plant extract or positive control substance. Ascorbic acid and BHT were used as positive controls. Background interferences from solvents were deducted from the activities of the corresponding extracts prior to calculating radical scavenging capacity as follows:
The antioxidant activity of plant extracts was expressed as IC50, which is defined as the concentration of extracts (in μ g/mL) required to scavenge 50% of DPPH radicals. IC50 values were estimated by a nonlinear regression (GraphPad Prism version 4.0). A lower IC50 value indicates higher antioxidant activity. The results are given as a mean ± standard deviation (SD) of experiments done in triplicate.
Antifungal activity
The fungus used in the assays was Rhizopus stolonifer (Ehrenger:Fries) Lind. var. stolonifer, and it was acquired from the Spanish Type Culture Collection (2344) preserved by the Department of Microbiology, School of Science, University of Valencia. Rhizopus stolonifer was grown on potato dextrose agar (PDA) medium on Petri plates (Ø 20 cm). The antimicrobial activity was evaluated by the method of mycelial growth (CitationZhang et al., 2006) using a 96 h culture at 25°C in darkness. Plant extracts were resuspended in sterile Eppendorf with DMSO and were added to sterile PDA medium. All extracts were assayed at a final concentration of 250 ppm. A 5-mm isolate of R. stolonifer mycelium was inoculated in fresh medium containing DMSO (control) and in fresh medium containing DMSO plus plant extract. After incubation for 24 h at 25°C in darkness, growth zones were measured with the Leica Q Win Program (v. 2.2) and converted into percentage of inhibition: [(Control − Treatment)/Control] × 100. The percentage of Rhizopus growth inhibition is expressed as a mean of four replicate tests. Benzoic and gallic acids were used as positive controls. Minimum inhibitory concentration (MIC) was determined by dilution of the extract to various concentrations (250–1000 ppm). MIC was defined as the lowest concentration of the extract that inhibited the growth for 24 h.
Results and Discussion
Antioxidant activity
We have studied several plant extracts belonging to different families to try to discover new applications and activities. The use of DPPH provides an easy and rapid way to evaluate antioxidant activity. DPPH is a free radical stable at room temperature, which produces a violet solution in methanol. In the presence of an antioxidant molecule, it is reduced giving rise to uncolored solutions. The mechanism involved in the reduction of DPPH free radicals is based on the capacity of some compounds to donate hydrogen. Some plants are rich in secondary metabolites derived from the shikimate pathway, such as, for example flavonoids, phenolic acids, and tannins. These phenolic compounds are able to donate hydrogen, presenting antiradical activity.
In the DPPH free radical scavenging capacity assay by TLC, the extracts that produced yellow spots on the plates were considered as antioxidants. The highest antioxidant/antiradical capacities were detected in the ethyl acetate, methanol, and aqueous extracts. After studying the plates sprayed with vanillin/H2SO4 and natural products reagents, it was deduced that the activity might be due mainly to phenolic compounds, flavonoids, and caffeic acids. The dichloromethane extracts barely showed antiradical capacity. These TLC tests were very useful to make a rapid screening of all the extracts.
The antioxidant activity was measured by calculating the IC50value. All the plants exhibited a concentration-dependent activity. Eight extracts presented IC50 values below 40 μ g/mL ( and ), showing good potential as free radical scavengers: ethyl acetate extract of Verbena officinalis L. (Verbenaceae); methanol extracts of Jasonia glutinosa L. (Asteraceae), Tanacetum parthenium Schultz (Asteraceae) flowers, Equisetum telmateia Ehrh (Equisetaceae), and Lythrum salicaria L. (Lythraceae); aqueous extracts of Jasonia glutinosa, Equisetum telmateia, and Lythrum salicaria. Among them, two extracts presented a higher antioxidant activity than did BHT (methanol extracts of Equisetum telmateia and Lythrum salicaria). Only Lyhtrum salicaria exhibited an outstanding in vitro antiradical capacity whose IC50 was almost the same as purified ascorbic acid, used as positive control ().
Figure 1 Antioxidant activity of the methanol extract of Lythrum salicaria, BHT, and ascorbic acid by the DPPH assay.
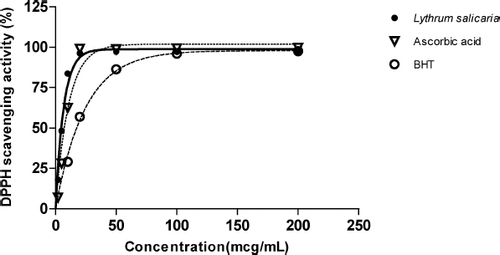
Table 2 Antioxidant activity (expressed as IC50) of Asteraceae extracts.
Table 3 Antioxidant activity (expressed as IC50) of miscellaneous extracts.
Although many plant extracts from the Asteraceae presented antioxidant activity by the DPPH method, none of them were higher than those of BHT and ascorbic acid. But for this, it is important to notice that the work has been carried out with crude extracts instead of pure substances. Jasonia glutinosa, popularly known in Spain as té de roca “rock tea” (CitationPardo de Santayana et al., 2005), showed antioxidant activity in all extracts. It grows on rocks from Spain, France, and Morocco, and it contains phenolic acids, flavonoids, sesquiterpene lactones, and essential oils (CitationBermejo et al., 2002). Tanacetum parthenium (feverfew) flowers presented a good result as a free radical scavenger. The antioxidant properties appear in the aqueous and methanol extracts of feverfew, being related with the presence of some flavonoids in its composition.
The other species from different families demonstrated antioxidant activity in some of their extracts, with Equisetum telmateia, Verbena officinalis, and Lythrum salicaria, more interesting than others; but the highest inhibitory effect on DPPH of all the plant extracts studied was due to the methanol extract of Lyhtrum salicaria. Purple loosestrife (L. salicaria) is used in traditional medicine for its astringent properties in common diarrhoea (CitationCoban et al., 2003) and contains anthocyanosides, flavonoids, phenolic acids, tannins, phthalates, sterols, and terpenes (CitationBecker et al., 2005). The methanol extract showed a very high antioxidant capacity, which might be due to the chemical composition, especially rich in polyphenolic compounds.
Antifungal activity
As far as the Asteraceae family is concerned (), ten extracts inhibited Rhizopus growth by more than over 20% compared with control plates containing fresh medium with DMSO: dichloromethane extracts of Anthemis arvensis L. subsp. arvensis, Anthemis cotula L., Cychorium intybus L., Jasonia glutinosa L., Santolina chamaecyparissus L. subsp. squarrosa Nyman, and Tussilago farfara L.; ethyl acetate extracts of Achillea millefolium L. subsp. millefolium leaves, Santolina chamaecyparissus susbp. squarrosa, and Tanacetum parthenium stem and leaves. The most effective was Anthemis arvensis and the least Jasonia glutinosa. From the rest of the families (), only the genus Anagallis showed a high inhibition of Rhizopus stolonifer. Values above 50% represent that the extracts might be a good alternative as additives to substitute synthesis products. (Benzoic and gallic acids, used as positive controls, produced 61.90% and 10.40% inhibition, respectively.)
Figure 2 Antifungal activity of Asteraceae extracts against Rhizopus stolonifer. Results are presented as means of four replicates.
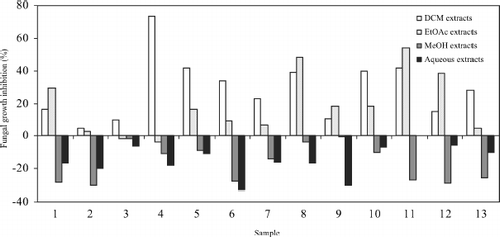
Figure 3 Antifungal activity of miscellaneous plant extracts against Rhizopus stolonifer. Results are presented as means of four replicates.
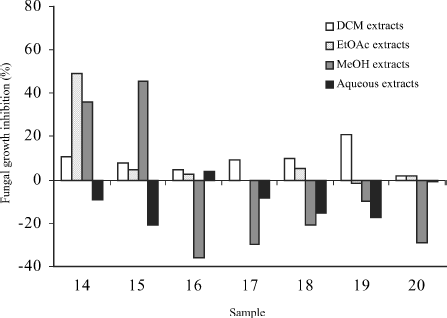
Rhizopus stolonifer was very sensitive to dichloro- methane extract of Anthemis arvensis subsp. arvensis (73% inhibition). Its antimicrobial effect seems to be related to the presence of sesquiterpene lactones, described in non-polar extracts of this plant (CitationVuckovic et al., 2006). The inhibition produced by the ethyl acetate herbal extracts of Tanacetum parthenium (54%) and Santolina chamaecyparissus (48%) may also be attributed to sesquiterpenes as they are frequent in many species of the Asteraceae (Bruneton, 1999). Both ethyl acetate and methanol extracts of Anagallis arvensis L. (Primulaceae) presented antifungal properties. This plant contains saponins that have already been reported as fungicidal active principles (CitationShoji et al., 1994; CitationAbdel-Gawad et al., 2000; CitationBruneton, 2000). The methanol extract of Anagallis foemina Miller (Primulaceae) also inhibited Rhizopus growth and saponosides might be the bioactive principles as composition is probably similar to A. arvensis. MIC data are given in .
Table 4 Minimun inhibitory concentration (MIC) of plant extracts against Rhizopus stolonifer
Conclusions
Different extracts obtained from selected Spanish plants were studied to determine their antioxidant and antifungal properties. According to our results, in most cases, the highest radical scavenger capacity was detected in methanol and aqueous extracts, which indicates that polyphenols may be responsible. The most interesting antioxidant activity, in order of effectiveness, was observed in extracts obtained from Lythrum salicaria, Equisetum telmateia, Jasonia glutinosa, Tanacetum parthenium and Verbena officinalis. The unexpected result of purple loosestrife, whose antiradical activity was similar to the reference substance ascorbic acid, note worthy. These species may be used as a source of natural antioxidants to stabilize food against oxidative deterioration or as biologically active products to protect cells from damage and senescence. On the other hand, the best results for the antifungal test against Rhizopus stolonifer were produced by nonpolar herbal extracts, with terpenoids being the active compounds responsible for the activity. Anthemis arvensis subsp. arvensis, Tanacetum parthenium, Santolina chamaecyparissus subsp. squarrosa, Anagallis arvensis, and Anagallis foemina had the strongest antifungal capacities.
The current study shows the potential of plants traditionally used in Spanish folk medicine to produce new active products with future applications as additives to preserve food from deterioration.
Acknowledgments
This work was funded by a grant from the University of Navarra Foundation. We also thank the Government of Navarra and the Alumni Navarrenses Association for the research fellowships for V.L. and S.A.
References
- MM Abdel-Gawad, SM El-Amin, H Ohigashi, Y Watanabe, N Takeda, H Sugiyama, and M Kawanaka. (2000). Molluscicidal saponins from Anagallis arvensis against schistosome intermediate hosts. Jpn J Infect Dis 53:17–19.
- NM Ansari, L Houlihan, B Hussain, and A Pieroni. (2005). Antioxidant activity of five vegetables traditionally consumed by South-Asian migrants in Bradford, Yorkshire, UK. Phytother Res 19:907–911.
- AK Bahceevli, S Kurucu, U Kolak, G Topcu, E Adou, and DG Kingston. (2005). Alkaloids and aromatics of Cyathobasis fruticulosa (Bunge) Aellen. J Nat Prod 68:956–958.
- H Becker, JM Scher, JB Speakman, and J Zapp. (2005). Bioactivity guided isolation of antimicrobial compounds from Lythrum salicaria. Fitoterapia 76:580–584.
- BP Bermejo, MJ Abad, AM Diaz, L Villaescusa, MA Gonzalez, and AM Silvan. (2002). Sesquiterpenes from Jasonia glutinosa: In vitro anti-inflammatory activity. Biol Pharm Bull 25:1–4.
- MS Blois. (1958). Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200.
- V Bondet, W Brand-Williams, and C Berset. (1997). Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. Lebensm Wiss Technol 30:609–615.
- W Brand-Williams, ME Cuvelier, and C Berset. (1995). Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 28:25–30.
- J Bruneton. Farmacognosia, Fitoquímica, Plantas medicinales, 2nd ed. España, Acribia, Zaragoza, (2000)615–626.
- C Cafarchia, N De Laurentis, MA Milillo, V Losacco, and V Puccini. (2002). Antifungal activity of essential oils from leaves and flowers of Inula viscosa (Asteraceae) by Apulian region. Parassitologia 44:153–156.
- Y Cai, Q Luo, M Sun, and H Corke. (2004). Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74:2157–2184.
- M Cascales. Estrés oxidativo. Envejecimiento y enfermedad. Instituto de España, Madrid, (1999)1–50.
- C Cavaleiro, E Pinto, MJ Goncalves, and L Salgueiro. (2006). Antifungal activity of Juniperus essential oils against dermatophyte, Aspergillus Candida strains. J Appl Microbiol 100:1333–1338.
- T Coban, GS Citoglu, B Sever, and M Iscam. (2003). Antioxidant activities of plants used in traditional medicine in Turkey. Pharm Biol 41:608–613.
- K Chen, GW Plumb, RN Bennett, and Y Bao. (2005). Antioxidant activities of extracts from five anti-viral medicinal plants. J Ethnopharmacol 96:201–205.
- AJ De Lucca, S Boue, MS Palmgren, K Maskos, and TE Cleveland. (2006). Fungicidal properties of two saponins from Capsicum frutescens and the relationship of structure and fungicidal activity. Can J Microbiol 52:336–342.
- HJ Dorman, M Kosar, K Kahlos, Y Holm, and R Hiltunen. (2003). Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J Agric Food Chem 51:4563–4569.
- RL Fernando, Z Varghese, and JF Moorhead. (1998). Differential ability of cells to promote oxidation of low density lipoproteins in vitro. Clin Chim Acta 269:159–173.
- T Finkel, and NJ Holbrook. (2000). Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247.
- B Frank, and S Gupta. (2005). A review of antioxidants and Alzheimer's disease. Ann Clin Psychiatry 17:269–286.
- SE Hankinson, MJ Stampfer, JM Seddon, GA Colditz, B Rosner, FE Speizer, and WC Willett. (1992). Nutrient intake and cataract extraction in women: A prospective study. Br Med j 305:335–339.
- MG Hertog, EJ Feskens, PC Hollman, MB Katan, and D Kromhout. (1993). Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 342:1007–1011.
- I Hinneburg, HJ Dorman, and R Hiltunen. (2006). Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem 97:122–129.
- DJ Huang, HJ Chen, W Hou, CD Lin, and YH Lin. (2006). Sweet potato (Ipomoeba batatas (L.) Lam “Tainong 57”) storage root mucilage with antioxidant activities in vitro. Food Chem 98:774–781.
- KFS Imaida, T Shivai, M Ohtani, K Nakanish, and N Ito. (1983). Promoting activities of butylated hydroxyanisole and butylated hydroxitoluene on 2-stage urinary bladder carcinogenesis and inhibition of gamma-glutamyl transpeptidase-positive foci development in the liver of rats. Carcinogenesis 4:895–899.
- D Ingram, K Sanders, M Kolybaba, and D Lopez. (1997). Case-control study of phyto-oestrogens and breast cancer. Lancet 350:990–994.
- OA Karabulut, U Arslan, and G Kuruoglu. (2004). Control of postharvest diseases of organically grown strawberry with preharvest applications of some food additives and postharvest hot water dips. J Phytopathol 152:224–228.
- M Mari, R Gregori, and I Donati. (2004). Postharvest control of Monilinia laxa Rhizopus stolonifer in stone fruit by peracetic acid. Postharvest Biol Technol 33:319–325.
- DJ Maron. (2004). Flavonoids for reduction of atherosclerotic risk. Curr Atheroscler Rep 6:73–78.
- J Martensson, R Steinherz, A Jain, and A Meister. (1989). Glutathione ester prevents buthionine sulfoximine-induced cataracts and lens epithelial cell damage. Proc Natl Acad Sci USA 86:8727–8731.
- KM Meepagala, G Sturtz, and DE Wedge. (2002). Antifungal constituents of the essential oil fraction of Artemisia dracunculus L. var. dracunculus. J Agric Food Chem 50:6989–6992.
- M Navab, AM Fogelman, JA Berliner, MC Territo, LL Demer, JS Frank, AD Watson, PA Edwards, and AJ Lusis. (1995). Pathogenesis of atherosclerosis. Am J Cardiol 76:18C–23C.
- J Northover, and T Zhou. (2002). Control of rhizopus rot of peaches with postharvest treatments of tebuconazole, fludioxonil, and Pseudomonas syringae. Can J Plant Pathol 24:144–153.
- de Pardo, M Santayana, E Blanco, and R Morales. (2005). Plants known as té in Spain: An ethno-pharmaco-botanical review. J Ethnopharmacol 98:1–19.
- BH Patterson, G Block, WF Rosenberger, D Pee, and LL Kahle. (1990). Fruit and vegetables in the American diet: Data from the NHANES II survey. Am J Public Health 80:1443–1449.
- PG Pietta. (2000). Flavonoids as antioxidants. J Nat Prod 63:1035–1042.
- MC Portillo-Ruiz, S Viramontes-Ramos, LN Munoz-Castellanos, MG Gastelum-Franco, and GV Nevarez-Moorillon. (2005). Antifungal activity of Mexican oregano (Lippia berlandieri Shauer). J Food Prot 68:2713–2717.
- MS Pyun, and S Shin. (2006). Antifungal effects of the volatile oils from Allium plants against Trichophyton species and synergism of the oils with ketoconazole. Phytomedicine 13:394–400.
- R Ross. (1993). The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 362:801–809.
- N Shoji, A Umeyama, K Yoshikawa, and S Arihara. (1994). Structures of anagallosaponins I-V and their companion substances from Anagallis arvensis L. Chem Pharm Bull 42:1750–1755.
- H Speisky, C Rocco, C Carrasco, EA Lissi, and C Lopez-Alarcon. (2006). Antioxidant screening of medicinal herbal teas. Phytother Res 20:462–467.
- UP Steinbrecher, HF Zhang, and M Lougheed. (1990). Role of oxidatively modified LDL in atherosclerosis. Free Radic Biol Med 9:155–168.
- Y Sun. (1990). Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med 8:583–599.
- I Vuckovic, L Vujisic, V Vajs, V Tesevic, S Macura, P Janackovic, and S Milosavljevic. (2006). Sesquiterpene lactones from the aerial parts of Anthemis arvensis L. Biochem Syst Ecol 34:303–309.
- H Wagner, and S Bladt. Plant Drug Analysis: A Thin Layer Chromatography Atlas. Springer-Verlag, Berlin, (1996)350–354.
- PYY Wong, and DD Kitts. (2006). Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem 97:505–515.
- N Yanishlieva. Inhibiting oxidationAntioxidants in Food: Practical ApplicationsJ Pokorny, N Yanishlieva, and M Gordon. CRC Press, Boca Raton, (2001)22–70.
- MM Zhang, YB Li, L Qi, and XC Wan. (2006). Antifungal activities of major tea leaf volatile constituents toward Colletorichum camelliae Massea. J Agric Food Chem 54:3936–3940.