Abstract
Microglia are activated in response to brain injury and release neurotoxic factors including nitric oxide (NO) and proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β). Lycopene, a potent antioxidant, is known to inhibit brain injury. In this study, we found that lycopene (5–20 μ M) significantly inhibited lipopolysaccharide (LPS)-induced NO release in primary cultured microglia. Lycopene (5–20 μM) also concentration-dependently diminished the LPS-induced production of proinflammatory cytokines such as TNF-α and IL-1β in microglia. Further study of the molecular mechanisms revealed that lycopene markedly inhibited extracellular signal-regulated kinase (ERK1/2) but not c-Jun N-terminal kinase (JNK1/2) or p38 mitogen-activated protein kinase (MAPK) phosphorylation stimulated by LPS in microglia. These results suggest that microglial inactivation by lycopene is at least partially due to activation of ERK1/2 phosphorylation Therefore, inhibition of NO and proinflammatory cytokine production in activated microglia by lycopene may represent a powerful and potential therapeutic strategy for various neurodegenerative diseases including ischemia-reperfusion cerebral infarction.
Introduction
Microglia are a type of neuroglia that support, nurture, and protect the neurons that maintain homeostasis of the fluid that bathes neurons (CitationGehrmann et al., 1995). It is an innate immune component of the central nervous system (CNS) parenchyma (CitationStreit, 2002). Under physiologic conditions, residential microglia are quiescent and scattered throughout the CNS. Occasionally, microglia are moderately activated to play the classic role as scavengers for the maintenance and restoration of the CNS. Activated microglia release proinflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α to induce inflammatory responses (CitationJohn et al., 2005). Furthermore, activated microglia also release neurotoxins like reactive oxygen species (ROS) and NO (CitationChao et al., 1992), which amplify the inflammatory responses and cause neuronal damage in the CNS. Sustained overactivation of microglia is found in many neurodegenerative diseases such as multiple sclerosis, Alzheimer's disease, Parkinson's disease, HIV-associated dementia, and ischemia-reperfusion brain injury (CitationGonzalez-Scarano & Baltuch, 1999; CitationKim & Joh, 2006).
Endotoxins are high-molecular-weight complexes of lipopolysaccharide (LPS) that are major components of the outer membranes of the cell walls of Gram negative bacteria (CitationBone, 1991). The most severe septic microvascular inflammatory responses, however, are caused by Gram negative bacteremia, and these responses can be produced by an injection of LPS. Endothelial injuries, activation of the coagulation cascade, platelet aggregation, and thrombocytopenia have all been shown to contribute to vascular fibrin deposition in LPS-induced septic shock (CitationBone, 1991; CitationSheu et al., 1994, Citation1999). LPS also triggers a series of inflammatory reactions in microglia. LPS was known to induce NO and TNF-αproduction in microglia through various extracellular signal-regulated kinases (ERKs), p38 mitogen-activated protein kinase (p38 MAPK), and c-Jun N-terminal kinases (JNKs) pathways (CitationBhat & Fan, 2002; CitationWaetzig et al., 2005).
Lycopene, found in tomatoes and other plants, is the most potent antioxidant among various common carotenoids (CitationDi Mascio et al., 1989). Dietary supplementation of lycopene resulted in a significant increase in serum lycopene levels and a reduction in the number of men who went on to develop prostate cancer in comparison with status-matched controls (CitationGuns & Cowell, 2005). The health benefits of lycopene might extend beyond fighting prostate cancer. Accumulating evidence suggests that the antiproliferative properties of lycopene may extend to other types of cancer (CitationGiovannucci et al., 2002). Furthermore, lycopene may also be useful in preventing heart disease. Lycopene apparently inhibits cholesterol synthesis and enhances low-density lipoprotein degradation (CitationRao, 2002).
In our previous study (CitationHsiao et al., 2004a), we found that the neuroprotective effect of lycopene, through its antioxidative property, mediates at least a portion of the free radical scavenging activity and inhibits microglia activation, resulting in a reduction in infarct volume in ischemia-reperfusion brain injury. However, the inhibitory effects of lycopene in LPS-induced inflammatory responses in microglia have still not yet been completely resolved. We therefore further examined the effect of lycopene in LPS-induced microglia activation and used the findings to further characterize the anti-inflammatory effect of lycopene.
Materials and Methods
Materials
Lycopene, LPS (Escherichia coli, serotype 0127:B8), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) were purchased from Sigma Chemical Co. (St. Louis, MO, USA); RPMI-1640 medium, fetal bovine serum (FBS), trypsin (0.25%), I-glutamine, and penicillin/streptomycin were from GibcoBRL (Gaithersburg, MD, USA); deoxyribonuclease type I (DNase I) was from Roche (Indianapolis, IN, USA); anti-ERK1/2, anti-phospho-ERK1/2 (Thr202/Tyr204), anti-JNK1/2, anti-p38 MAPK, and anti-phospho-JNK1/2 (Thr183/Tyr185) monoclonal antibodies (mAbs) were from Cell Signaling (Beverly, MA, USA); anti-phospho-p38 MAPK (Ser182) mAb was from Santa Cruz (Santa Cruz, CA, USA); horseradish peroxidase–conjugated secondary antibody was from Amersham (Buckinghamshire, UK); and IL-1β and TNF-α nzyme immunoassay (EIA) kits were from R&D Systems (Minneapolis, MN, USA). Lycopene was dissolved in 0.1% DMSO. In this study, a vehicle solvent control was always included.
Cell cultivation
Wistar rats (7 days old; from the Experimental Animal Center of the College of Medicine, National Taiwan University) were used in this study. All animal experiments and care was performed according to the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, DC, 1996). Wistar rats were deeply anesthetized with ether and transcardially perfused with normal saline until the lungs and liver were clear of blood. After perfusion, the brain was removed and kept in RPMI-1640 medium. After dissecting the meninges, the brain tissue was minced in ice-cold RPMI-1640 and treated with trypsin (0.25%) and deoxyribonuclease (10 mg/mL) in RPMI-1640 for 2 h at 37°. The treated tissues were further minced in 10% FBS and centrifuged at 1000 rpm for 10 min. The tissue pellet was resuspended in RPMI-1640 and then seeded in 75 cm2 flasks at 37°C (95% O2, 5% CO2).
Microglia were harvested from flasks of mixed glial cultures by shaking for 2 h. Cells were collected by centrifugation and then seeded at 5 × 105 cells/mL. After incubation for 1 h at 37°C, nonadherent or weakly adherent cells were removed by gentle shaking and washed out. The cells were further cultured in RPMI-1640 supplemented with 10% FBS for 1 day. Approximately 2 × 106 cells were obtained per brain used (CitationHsiao et al., 2004a). To determine the purity of the microglia, immunocytochemical analysis was carried out using a microglial-specific OX-42 antibody. These primary culture cells were > 95% OX-42–positive, indicating they were composed of microglia.
Cell viability
Microglia viability after 24 h of continuous exposure to different concentrations of LPS (1–200 ng/mL) and lycopene (1–20 μ M) was measured with a colorimetric assay based on the ability of mitochondria in viable cells to reduce the MTT as previously described (CitationMosmann, 1983). The percentage of cell viability was calculated as the absorbance of treated cells/control cells × 100%.
Determination of nitrite concentration
To determine NO production from microglia, nitrite (a stable oxidative end-product of NO) accumulation in the media of microglia was measured using a colorimetric method (CitationGreen et al., 1982), with minor modification. Briefly, 100 μ L supernatant was incubated with an equal volume of Griess reagent (1% sulfanilamide and 0.1% naphthyl-ethylenediamine dihydrocholoride in 2.5% phosphoric acid). After a 30-min incubation at room temperature, the optical absorbance at 550 nm was measured with a microplate reader. Nitrite concentrations were calculated by regression with standard solutions of sodium nitrite prepared in the same culture medium (CitationHsiao et al., 2004b).
Enzyme-linked immunosorbent assay (ELISA)
For determination of IL-1β and TNF-αproduction from microglia, microglia (5 × 105 cells/mL) were plated onto 24-well culture plates for 24 h. Cells were pretreated with various concentrations of lycopene (5, 10, and 20 μ M) or an isovolumetric solvent control (0.1% DMSO) for 30 min and then treated with LPS (100 ng/mL) for the indicated times (1–24 h). After incubation with LPS, supernatants were collected and immediately frozen at −70°C. The IL-1β and TNF-α levels of the supernatants were measured using ELISA kits according to the manufacturer's protocol.
Western blot analysis
For determination of the expression of MAPKs in microglia, Western blot analyses were performed as described previously (CitationHsiao et al., 2004b). Microglia (5 × 105cells/mL) were cultured on 24-well plates and treated with lycopene (5, 10, and 20 μ M) or an isovolumetric solvent control for 30 min followed by the addition of LPS (100 ng/mL). At indicated times, cells were washed with ice-cold PBS buffer (pH 7.3). Proteins were extracted with lysis buffer for 30 min. In addition, phosphatase inhibitors (10 mM sodium fluoride, 1 mM sodium orthovanadate, and 10 mM sodium pyrophosphate) were added to the lysis buffer for the phosphorylated MAPK analysis. Lysates were centrifuged, and the supernatant (50 μ g protein) was subjected to SDS-PAGE and electrophoretically transferred onto PVDF membranes (0.45 mm; Hybond-P; Amersham). After incubation in blocking buffer (50 mM Tris-HCl, 100 mM NaCl, 0.1% Tween 20, and 5% dry skim milk; pH 7.5) overnight at 48 h and being washed three-times with PBS buffer, blots were treated with either anti-phospho-ERK1/2 (p42/44), anti-ERK1/2, anti-phospho-JNK1/2 (p46/54), anti-JNK1/2, anti-p38 MAPK, or anti-phospho-p38 MAPK mAbs (1:2000) in a PBS buffer for 3 h. They were subsequently washed three-times with PBS buffer and incubated with peroxidase-conjugated goat anti-mouse or anti-rabbit antibody (1:3000) for 2 h. Blots were then washed three-times, and the band with peroxidase activity was detected using film exposure with enhanced chemiluminescence detection reagents (ECL+ System; Amersham). Densitometric analysis of specific bands was performed using a Photo-Print Digital Imaging System (IP-008-SD) with analytic software (Bio-1Dlight, V 2000).
Statistical analysis
The experimental results are expressed as the means ± SEM and are accompanied by the number of observations. Data were assessed by the method of analysis of variance (ANOVA). If a significant difference among the group means was noted, the difference between two groups was assessed using the Newman-Keuls method. A p value of less than 0.05 was considered statistically significant.
Results
Effect of lycopene on nitrite production in LPS-induced microglia activation
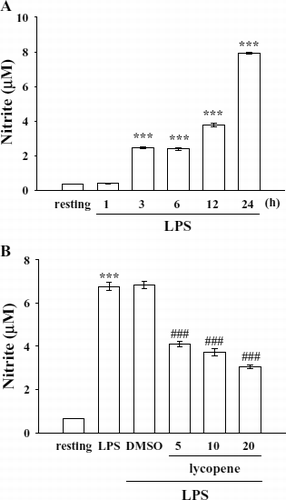
According to a preliminary test, activation of microglia by LPS (100 ng/mL) induced a significant and marked increase in nitrite formation. Therefore, an LPS concentration of 100 ng/mL was employed in the following experiments. In this study, the concentration of nitrite produced in the cell supernatant time-dependently increased from 0.4 ± 0.0 (resting) to 7.9 ± 0.1 μ M at 24 h after LPS activation (). Lycopene (5–20 μ M) markedly inhibited nitrite production stimulated by LPS (100 ng/mL) by approximately 43.9%, 50.1%, and 60.9%, respectively (). Lycopene neither interfered with the Griess reaction nor reacted with native NO (data not shown). These results demonstrate that lycopene markedly suppresses NO production stimulated by LPS in microglia. Furthermore, neither LPS (100 ng/mL) nor the solvent control (0.1% DMSO) affected the cell viability of microglia for 24 h according to the MTT assay (data not shown). Therefore, cells were treated with LPS at 100 ng/mL for 24 h in the following experiments.
Figure 1 Effect of lycopene on nitrite formation in LPS-activated microglia. Microglia (5 × 105 cells/mL) were treated with (A) LPS (100 ng/mL) at the indicated times (1–24 h) OR (B) various concentrations of lycopene (5, 10, and 20 μ M) or an isovolumetric solvent control (0.1% DMSO) for 30 min, followed by the addition of LPS (100 ng/mL). Cell-free supernatants were assayed for nitrite production as described in “Materials and Methods.” Data are presented as the means ± SEM (n = 4). ***p < 0.001 compared with the resting group; # # #p < 0.001 compared with the LPS-treated group.
Effect of lycopene on IL-1β production in LPS-induced microglia activation
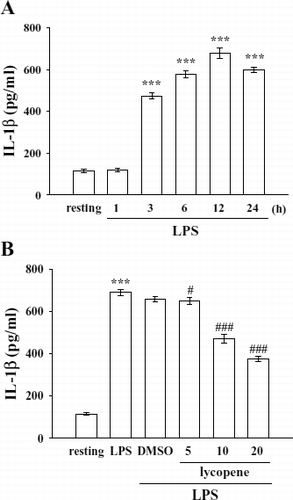
LPS (100 ng/mL) induced a time-dependent increase in IL-1β formation in microglia, and it reached a maximal level at 12 h rising from 114.4 ± 9.1 (resting) to 678.6 ± 25.7 pg/mL (). On the other hand, after pretreatment of cells with various concentrations of lycopene (5–20 μ M) for 30 min followed by the addition of LPS (100 ng/mL) for 12 h, we found that lycopene (5, 10, and 20 μ M) concentration-dependently inhibited IL-1β production by approximately 7.1%, 38.2%, and 54.9%, respectively ().
Figure 2 Effect of lycopene on IL-1β production in LPS-activated microglia. Microglia (5 × 105cells/mL) were treated with (A) LPS (100 ng/mL) at the indicated times (1–24 h) OR (B) various concentrations of lycopene (5, 10, and 20 μ M) or an isovolumetric solvent control (0.1% DMSO) for 30 min, followed by the addition of LPS (100 ng/mL). Cell-free supernatants were assayed for IL-1β production as described in “Materials and Methods.” Data are presented as the means ± SEM. (n = 4). ***p < 0.001 compared with the resting group; # p < 0.05 and # # #p < 0.001 compared with the LPS-treated group.
Effect of lycopene on TNF-α production in LPS-induced microglia activation
Activation of microglia by LPS (100 ng/mL) induced a significant increase in TNF-α formation at the indicated times (1–6 h) (resting, 1046.0 ± 40.2 pg/mL; 3 h, 2267.5 ± 77.2 pg/mL) (). The peak activation of TNF-α formation occurred 1∼ 3 h after LPS (100 ng/mL) timulation, and it had returned to basal levels after 12 h ().
Figure 3 Effect of lycopene on TNF-α production in LPS-activated microglia. Microglia (5 × 105 cells/mL) were treated with (A) LPS (100 ng/mL) at the indicated times (1–24 h) or (B) various concentrations of lycopene (5, 10, and 20 μ M) or an isovolumetric solvent control (0.1% DMSO) for 30 min, followed by the addition of LPS (100 ng/mL). Cell-free supernatants were assayed for TNF-α production as described in “Materials and Methods.” Data are presented as the means ± SEM. (n = 4). ***p < 0.001 compared with the resting group; # # #p < 0.001 compared with the LPS-treated group.
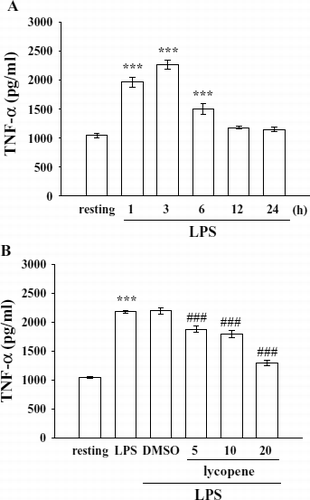
After pretreatment of cells with various concentrations of lycopene (5–20 μ M) for 30 min followed by the addition of LPS (100 ng/mL) for 3 h, lycopene (5, 10, and 20 μ M) significantly inhibited TNF-α production by approximately 26.7%, 34.1%, and 78.4%, respectively ().
Effects of lycopene on ERK1/2, JNK1/2, and p38 MAPK phosphorylation in LPS-induced microglia activation
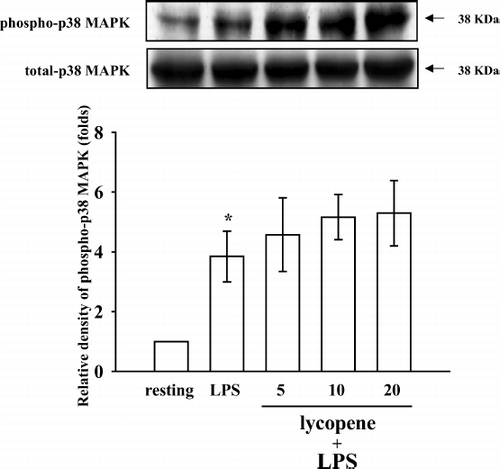
To further investigate the inhibitory mechanisms of lycopene in LPS-induced microglia activation, several signaling molecules were detected including p38 MAPK, ERK1/2, and JNK1/2. The immunoblotting analysis revealed that treatment with LPS (100 ng/mL) induced rapid and markedly time-dependent phosphorylation of ERK1/2 (p42/p44), JNK1/2 (p46/p54), and p38 MAPK, which reached maximal levels at approximately 45 min and then returned to the basal level (data not shown). After being pretreated with lycopene (5, 10, and 20 μ M) for 30 min, phosphorylated ERK1/2 stimulated by LPS (100 ng/mL) was markedly inhibited by lycopene in a concentration-dependent manner (). At a higher concentration of 20 μ M, lycopene inhibited ERK1/2 phosphorylation by approximately 51% (). However, neither JNK1/2 () nor p38 MAPK () phosphorylation stimulated by LPS (100 ng/mL) was significantly inhibited by lycopene (5, 10, and 20 μ M).
Figure 4 Effect of lycopene on ERK1/2 phosphorylation in LPS-activated microglia. Microglia (5 × 105 cells/mL) were treated with various concentrations of lycopene (5, 10, and 20 μ M) or an isovolumetric solvent control (0.1% DMSO) for 30 min, followed by the addition of LPS (100 ng/mL). ERK1/2 phosphorylation was determined by Western blotting with a monoclonal antibody that recognizes only phosphorylated ERK1/2 (p42/p44). Data are presented as the means ± SEM. (n = 3). ***p < 0.001 compared with the resting group; # p < 0.05 and # #p < 0.01 compared with the LPS-treated group.
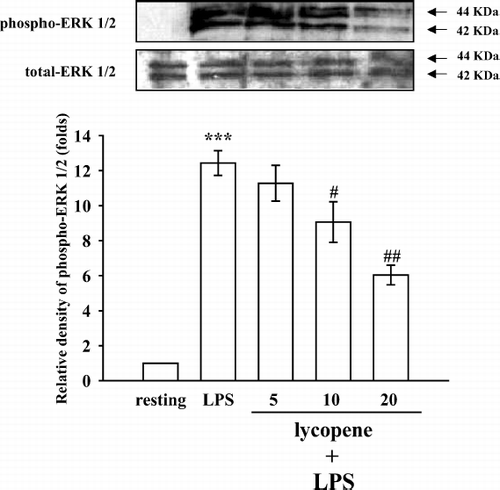
Figure 5 Effect of lycopene on JNK1/2 phosphorylation in LPS-activated microglia. Microglia (5 × 105 cells/mL) were treated with various concentrations of lycopene (5, 10, and 20 μ M) or an isovolumetric solvent control (0.1% DMSO) for 30 min, followed by the addition of LPS (100 ng/mL). JNK1/2 phosphorylation was determined by Western blotting with a monoclonal antibody that recognizes only phosphorylated JNK1/2 (p46/p54). Data are presented as the means ± SEM. (n = 3). ***p < 0.01 compared with the resting group.
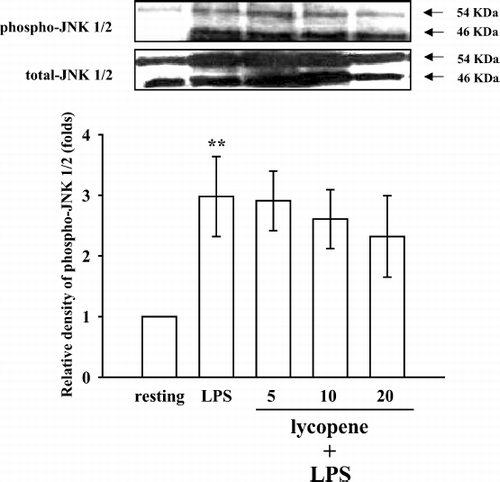
Figure 6 Effect of lycopene on p38 MAPK phosphorylation in LPS-activated microglia. Microglia (5 × 105 cells/mL) were treated with various concentrations of lycopene (5, 10, and 20 μ M) or an isovolumetric solvent control (0.1% DMSO) for 30 min, followed by the addition of LPS (100 ng/mL). p38 MAPK phosphorylation was determined by Western blotting with a monoclonal antibody that recognizes only phosphorylated p38 MAPK. Data are presented as the means ± SEM. (n = 3). * p < 0.05 compared with the resting group.
Discussion
In this study, we demonstrate an anti-inflammatory effect of lycopene in primary cultured microglia. Lycopene markedly inhibited proinflammatory cytokines, such as TNF-α and IL-1β and NO in LPS-stimulated microglia.
It is known that microglia play a key role in mediating inflammatory processes in the CNS, which are associated with various neurodegenerative diseases. LPS, a glycolipid derived from the membrane surface of Gram-negative bacteria (endotoxin), can trigger a series of inflammatory reactions in phagocytes such as microglia. With LPS stimulation, microglia are activated to drastically change their cellular functions, producing various types of inflammatory mediators such as NO, TNF-α, and IL-1β. NO and TNF-α are two major inflammatory mediators. NO is beneficial as a messenger or modulator, but in conditions such as oxidative stress, it is potentially toxic. NO generation by activated microglia has been shown to cause excitotoxicity through inducing glutamate release and inhibiting neuronal respiration (CitationBal-Price & Brown, 2001). TNF-α is a potent inducer of IL-1β Jongeneel, 1995). TNF-α and IL-1β released from activated microglia also stimulate NO production in glial cells and may have a direct effect on neurons through activating receptors that contain the death domains involved in apoptosis (CitationHirsch et al., 2003).
Lycopene has been demonstrated to be the most potent antioxidant, and various antioxidants are ranked as follows: lycopene > α -tocopherol > α -carotene > β -cryptoxanthin > zeananthin = β -carotene > lutein (CitationHeber & Lu, 2002). The consumption of 25 g of tomato puree (containing 7 mg of lycopene) for 14 consecutive days increased plasma and lymphocyte carotenoid concentrations, and this was related to an improvement in lymphocyte resistance to oxidative stress (CitationPorrini & Riso, 2000). Therefore, lycopene absorbed from tomato products may act as an in vivo antioxidant (CitationHeber & Lu, 2002). In this study, lycopene (5–20 μ M) markedly inhibited the inflammatory response stimulated by LPS in microglia. Inhibition of TNF-α by lycopene may be associated with lycopene-mediated suppression of IL-1β. Our results are also consistent with a previous report finding that lycopene could inhibit NO production in activated microglia, and regular consumption of a tomato-based drink including lycopene reduced the TNF-α content of blood (CitationRiso et al., 2006).
MAPKs play important roles in mediating cytokine (i.e., TNF-α and IL-1β) release in LPS-stimulated microglia activation (CitationBhat & Fan, 2002; CitationNakajima et al., 2004; CitationWaetzig et al., 2005). Therefore, we further investigated the roles of MAPKs involved in lycopene-mediated suppression of cytokine release (i.e., TNF-α and IL-1β) and NO formation in LPS-stimulated microglia. MAPKs are a family of serine-threonine kinases activated by many stimuli including growth factors and hormones in proliferative cells (CitationBugaud et al., 1999). MAPKs are able to regulate a number of transcription factors, cytoplasmic proteins, and downstream kinases. This family consists of three major subgroups. The ERKs are involved in proliferation, adhesion, and cell progression (CitationBugaud et al., 1999). p38 MAPK and JNKs or stress-activated protein kinases (SAPKs), which include the 46-kDa JNK1 and 54-kDa JNK2 isoforms, are involved in death signaling processes (CitationBugaud et al., 1999). Lycopene (10 μ M) was shown to inhibit LPS-induced MAPKs (i.e., ERK1/2, JNK1/2, and p38 MAPK) phosphorylation in murine dendritic cells (CitationKim et al., 2004). However, our results showed that lycopene only inhibited ERK1/2, not JNK1/2 or p38 MAPK phosphorylation, in LPS-stimulated microglia. This discrepancy needs to be further investigated.
In conclusion, our results suggest that lycopene inhibition of LPS-induced NO, IL-1β, and TNF-α formations may be mediated, at least in part, by inhibition of ERK1/2 phosphorylation in primary cultured microglia. Lycopene is a naturally occurring material with few adverse effects; thus, treatment with lycopene may represent a novel approach for improving function in neurodegenerative diseases.
Acknowledgments
This work was supported by grants from the National Science Council of Taiwan (94-2321-B-038-001), Shin Kong Wu Ho-Su Memorial Hospital (SKH-TMU-92-27), and Min-Sheng Healthcare (93MSH-TMU-10), and by a Topnotch Stroke Research Center Grant, Ministry of Education, Taiwan.
References
- A Bal-Price, and GC Brown. (2001). Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci 21:6480–6491.
- NR Bhat, and F Fan. (2002). Adenovirus infection induces microglial activation: Involvement of mitogen-activated protein kinase pathways. Brain Res 948:93–101.
- RC Bone. (1991). The pathogenesis of sepsis. Ann Int Med 115:457–469.
- F Bugaud, F Nadal-Wollbold, S Levy-Toledano, JP Rosa, and M Bryckaert. (1999). Regulation of c-jun-NH2 terminal kinase and extracellular-signal regulated kinase in human platelets. Blood 94:3800–3805.
- CC Chao, S Hu, TW Moltor, EG Shaskan, and PK Peterson. (1992). Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol 149:2736–2741.
- P Di Mascio, S Kaiser, and H Sies. (1989). Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys 274:532–538.
- J Gehrmann, Y Matsumoto, and GW Kreutzberg. (1995). Microglia: Intrinsic immuneffector cell of the brain. Brain Res Rev 20:269–287.
- E Giovannucci, EB Rimm, Y Liu, MJ Stampfer, and WC Willett. (2002). A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst 94:391–398.
- F Gonzalez-Scarano, and G Baltuch. (1999). Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci 22:219–240.
- LC Green, DA Wagner, J Glogowski, PL Skipper, JS Wishnok, and SR Tannenbaum. (1982). Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126:131–138.
- ES Guns, and SP Cowell. (2005). Drug insight: Lycopene in the prevention and treatment of prostate cancer. Natl Clin Pract Urol 2:38–43.
- D Heber, and Y Lu. (2002). Overview of mechanisms of action of lycopene. Exp Biol Med 227:920–923.
- EC Hirsch, T Breidert, E Rousselet, S Hunot, A Hartmann, and PP Michel. (2003). The role of glial reaction and inflammation in Parkinson's disease. Ann NY Acad Sci 991:214–228.
- G Hsiao, TH Fong, NH Tzu, KH Lin, DS Chou, and JR Sheu. (2004a). A potent antioxidant, lycopene, affords neuroprotection against microglia activation and focal cerebral ischemia in rats. In Vivo 18:351–356.
- G Hsiao, HY Huang, TH Fong, MY Shen, CH Lin, CM Teng, and JR Sheu. (2004b). Inhibitory mechanisms of YC-1 and PMC in the induction of iNOS expression by lipoteichoic acid in RAW 264.7 macrophages. Biochem Pharmacol 67:1411–1419.
- GR John, SC Lee, X Song, M Rivieccio, and CF Brosnan. (2005). IL-1-regulated responses in astrocytes: Relevance to injury and recovery. Glia 49:161–176.
- CV Jongeneel. (1995). Transcriptional regulation of the tumour necrosis factor α gene. Immunology 193:210–216.
- GY Kim, JH Kim, SC Ahn, HJ Lee, DO Moon, CM Lee, and YM Park. (2004). Lycopene suppresses the lipopolysaccharide-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and nuclear factor-kappa B. Immunology 113:203–211.
- YS Kim, and TH Joh. (2006). Microglia, major player in the brain inflammation: Their roles in the pathogenesis of Parkinson's disease. Exp Mol Med 38:333–347.
- T Mosmann. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 85:55–63.
- K Nakajima, Y Tohyama, S Kohsaka, and T Kurihara. (2004). Protein kinase C alpha requirement in the activation of p38 mitogen-activated protein kinase, which is linked to the induction of tumor necrosis factor alpha in lipopolysaccharide-stimulated microglia. Neurochem Int 44:205–214.
- M Porrini, and P Riso. (2000). Lymphocyte lycopene concentration and DNA protection from oxidative damage is increased in women after a short period of tomato consumption. J Nutr 130:189–192.
- AV Rao. (2002). Lycopene, tomatoes, and the prevention of coronary heart disease. Exp Mol Med 227:908–913.
- P Riso, F Visioli, S Grande, S Guarnieri, C Gardana, P Simonetti, and M Porrini. (2006). Effect of a tomato-based drink on markers of inflammation, immunomodulation, and oxidative stress. J Agric Food Chem 54:2563–2566.
- JR Sheu, SH Chao, MH Yen, and TF Huang. (1994). In vivo antithrombotic effect of triflavin, an Arg-Gly-Asp containing peptide on platelet plug formation in mesenteric microvessels of mice. Thromb Haemost 72:617–621.
- JR Sheu, WC Hung, CH Wu, MC Ma, YC Kan, CH Lin, MS Lin, HN Luk, and MH Yen. (1999). Reduction in lipopolysaccharide-induced thrombocytopenia by triflavin in a rat model of septicemia. Circulation 99:3056–3062.
- WJ Streit. (2002). Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 40:133–139.
- V Waetzig, K Czeloth, U Hidding, K Mielke, M Kanzow, S Brecht, M Goetz, R Lucius, T Herdegen, and UK Hanisch. (2005). c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia 50:235–246.