Abstract
Sequential extraction with petroleum ether, chloroform–ethyl acetate, methanol, and water was carried out on 40 commonly used Chinese medicinal herbs. The antioxidant power of different fraction extracts was measured with both the DPPH assay and the FRAP assay. Ascorbic acid and butylated hydroxytoluene were used as two reference antioxidants. The total phenolic content of the extracts was measured by Folin-Ciocalteu method. The fraction extracts showed different antioxidant activity in the two assays; however, there was a significant positive correlation between the DPPH assay and the FRAP assay. Methanol fraction extracts generally demonstrated stronger antioxidant power and higher total phenolic content than did the others. The results suggest that Radix Sanguisorbae, Cortex cinnamomi, Herba taxilli, Semen arecae, and L., Cinnamomum cassia Presl ., Taxillus sutchuenensis (Lecomte) Danser, Areca catechu Linn. and Scutellaria baicalensis Georgi contain relatively high values in antioxidant power and/or high total phenolic content. This study is the first comprehensive antioxidant activity evaluation among Chinese medicinal herbs. It demonstrates that most Chinese medicinal herbs are also useful in preventing various degenerative diseases.
Introduction
Worldwide there are 5.8 × 108 people aged over 65. This number is predicted to increase to beyond 10 × 108 by 2020 (CitationZhang & Yu, 2000). Consequently, aging and age-related degenerative diseases, such as cancer, cardiovascular disease, Alzheimer's disease, and Parkinson's disease, have become important and inescapable socioeconomic and health care issues (CitationSun et al., 1999; CitationParejo et al., 2002). Aging and age-related diseases are associated with oxidative stress. Oxidative stress is a pro-oxidant shift in the oxidant:antioxidant balance of the body, caused by a relative or absolute deficiency of antioxidants (CitationGutteridge & Halliwell, 1994; CitationSun et al., 1999; CitationParejo et al., 2002). Cells can tolerate mild oxidative stress; however, severe oxidative stress results in, for example, DNA damage, lipid peroxidation, and protein damage. These changes are believed to lead to age-related disease, disability, and death (CitationGutteridge & Halliwell, 1994; CitationLiu, 2001; CitationChoi et al., 2002).
Consumption of fruits and vegetables is associated with a lowered risk of cancer, cardiovascular diseases, and other age-related diseases (CitationBlock et al., 1992). Various medicinal herbs, which are reported to be effective in treating aging, carcinoma and cardiovascular disease in animals, are reported to have high antioxidant power (CitationCarini et al., 2001; CitationPrakash et al., 2001; CitationSaleem et al., 2001; CitationSharma et al., 2001; CitationVohra et al., 2001; CitationYan et al., 2006). Their protective effect has been ascribed in part to the antioxidants vitamin C, vitamin E, polyphenols, carotenoids, and other active ingredients in plant based foods. An antioxidant has power to scavenge and remove reactive oxygen species or reduce oxidative substances in order to protect tissues and organs from oxidative damage. Therefore, crude extracts of fruits, herbs, vegetables, cereals, and other plant foods rich in antioxidant molecules have received a growing interest for promoting healthy life. Many regularly used Chinese medicinal herbs, which contain high antioxidant components such as vitamin E, flavonoids, phenolic acids, carotenoids, ascorbic acid, and glutathionine, have drawn attention (CitationLarson, 1988). However, there are few comprehensive reports about the antioxidant activities of commonly used traditional Chinese medicinal herbs.
There are several methods for measuring the “antioxidant activity” of foods and medicinal herbs. These include the N,N-dimethyl-p-phenylendiamine assay (DMPD assay) (CitationFogliano et al., 1999; CitationSchlesier et al., 2002), the 1,1-diphenyl-2-picrylhydrazyl assay (DPPH assay) (CitationBondet et al., 1997; CitationFauconneau et al., 1997), the Trolox equivalent antioxidant capacity assay (TEAC assay) (CitationMiller et al., 1996; CitationRe et al., 1999), the total radical-trapping antioxidant parameter assay (TRAP assay) (CitationWayner et al., 1985; CitationGhiselli et al., 1995), and the ferric reducing/antioxidant power assay (FRAP assay) (CitationBenzie & Strain, 1996, Citation1999). There are also methods that measure inhibition of lipid peroxidation, such as the peroxide value assay (POV assay) (CitationSteger & Mühlebach, 1997) and the 2-thiobarbituric acid assay (TBA assay) (CitationCoudray et al., 1995; CitationGuillén-Sans & Guzmán-Chozas, 1998). These methods have been used to measure antioxidant power or effects of pure chemicals, fruit juices, extracts of medicinal plants, teas, fruits, or vegetables.
In this study, extracts of 40 commonly used Chinese medicinal herbs were investigated for their antioxidant power in order to identify those herbs that may be most useful for follow-up study in terms of potential therapy to oppose oxidative stress and promote healthy aging. For screening of a large quantity of samples, a simple, rapid, reliable, and sensitive method is needed. The DPPH assay and FRAP assay fit these requirements and need only small amounts of sample (CitationBenzie et al., 1999; CitationChoi et al., 2002). Additionally, the Folin-Ciocalteu method was used for assaying total phenolic content of the herbal extracts. The results of this initial screening of antioxidant activity for the 40 herbs tested were compared with those of two commonly used reference antioxidants, butylated hydroxytoluene (BHT) and ascorbic acid.
Materials and Methods
Reagents
Steinheim,1,1-Diphenyl-2-picrylhydrazyl (DPPH: Sigma-Aldrich, Steinheim, Germany),2,4,6,-tripyridyl-S-triazine (TPTZ; Fluka, Buchs, Switzerland), Folin-Ciocalteu's reagent (BDH, Poole, England), gallic acid (Advanced Technology and Industrial Co. Ltd, Hong Kong), ascorbic acid (Panreac Quimica SA, Barcelona, Spain) and butylated hydroxytoluene (BHT; Acros Organics, New Jersey, USA) were used in this study. All the other reagents were of analytical grade (Lab-Scan Analytical Sciences, Bangkok, Thailand).
Chinese medicinal herbs and extracts
A total of 40 common Chinese medicinal herbs () were selected for screening. They were provided by the Laboratory of Traditional Chinese Medicine of the Institute of Modern Chinese Medicine, Institute of Materia Medica of The Hong Kong Polytechnic University. The voucher specimens were stored in dark at ∼ 20°C in plastic bags in Dr. Peter H.F. Yu's laboratory (Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University). The herbs were cut into small pieces, freeze-dried (24–48 h), weighed, and finally ground with a blender into powder form. The herbal powders were passed through a 12-mesh sieve and subjected to a sequential extraction with petroleum ether, chloroform–ethyl acetate, methanol, and water as shown in . For each sample, herbal powder (100 g) was extracted three times for 24 h in dark at room temperature with various solvents (1 L). Filtrates from the same solvent were pooled together for subsequent drying. The extracts from petroleum ether, chloroform–ethyl acetate, and methanol, were dried in a vacuum drier at 30°C and 0.07 MPa, whereas the extracts from water were freeze-dried (24–48 h). Therefore, four fraction extracts—petroleum ether fraction (PEF), chloroform–ethyl acetate fraction (CEF), methanol fraction (MF); and water fraction (WF)—were obtained for each herb. They were stored at −20°C until use. The dried samples of the different solvent fractions of each herb were redissolved in water, methanol, or ethanol to prepare solutions of different concentrations for the following assays.
Figure 1 Sequential extraction method of dried Chinese medicinal herbs by petroleum ether, chloroform–ethyl acetate, methanol, and water.
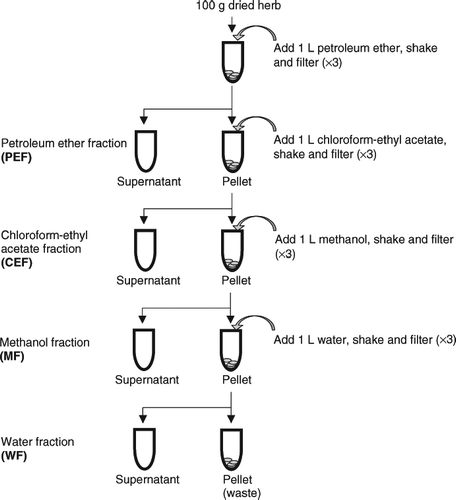
Table 1 The percentage DPPH scavenging capacity (SR%) of the petroleum ether, chloroform–ethyl acetate, methanol and water fractions of the Chinese medicinal herbs.
DPPH assay
In this assay, antioxidants in the sample scavenge the 1,1-diphenyl-2-picrylhydrazyl free radical (DPPH). This radical has a maximal absorption at 515 nm, and scavenging results in a drop in absorbance, the size of which is proportional to the antioxidant capacity of the sample. In brief, the absorbance value of 1.95 mL of DPPH (24 mg/L) in methanol was measured as control, then 50 μ L of each extract solution at a concentration of 7.5 mg/mL was mixed, and the absorbance change was measured using a UV-visible spectrometer (PerkinElmer Lambda 35, Massachusetts, USA) at 515 nm. The change in absorbance was determined at 2, 3, 4, and 5 min, and then at 5 min intervals until the absorbance change was not more than 0.003 absorbance units/min. The final absorbance reading of the sample was used to calculate the percentage free radical scavenging capacity (SR%) as follows:
Where Acontrol is the absorbance value of the DPPH solution before addition of herbal fraction extract solution or reference antioxidants solution, and Asample is the final absorbance value of DPPH solution after addition of herbal fraction extract solution or reference antioxidants solution.
For the fraction extracts of an SR% beyond 50% at 7.5 mg/mL, the SR% of several different concentrations was measured. The SR% values were plotted against the fraction extract concentrations, and a linear regression curve was established to obtain the SC50, which is the concentration of sample necessary to decrease by 50% the absorbance of DPPH. Also, freshly made BHT methanol solution and aqueous ascorbic acid solution of different concentrations were assayed for their SR% to determine the SC50 value. The results were expressed as radical scavenging efficiency (SE), which is 1000-fold the inverse of the SC50 value.
FRAP assay
The FRAP assay is a direct test of “total antioxidant power,” which uses antioxidants as reductants in a redox-linked colorimetric method, employing an easily reduced oxidant, ferric ion (Fe3 +), presents in stoichiometric excess (CitationFauconneau et al., 1997). At low pH, the ferric tripyridyltriazine (Fe3 +-TPTZ) complex is reduced by antioxidants in the sample to the ferrous form (Fe2 +-TPTZ), which appears an intense blue color. The reaction can be monitored at 593 nm. The assay was performed as described by CitationBenzie et al. (1996) using a Cobas Fara centrifugal analyzer (Roche Diagnostics Ltd., Basel, Switzerland). In brief, 300 μ L freshly prepared FRAP reagent [25 mL acetate buffer (300 mmol/L, pH 3.6), 2.5 mL TPTZ (10 mmol/L) in 40 mmol/L HCl, and 2.5 mL FeCl3· 6H2O solution (20 mmol/L) were mixed as required to prepare working FRAP reagent] was warmed to 37oC, and a reagent blank reading was taken at 593 nm; 10 μ L of each fraction extract solution of an appropriate concentration was then added along with 30 μ L H2O. The 0–4 min absorbance change (Δ A593 nm) was calculated for each sample and related to Δ A593 nm of a Fe2 + standard solution tested in parallel to obtain the FRAP value of each sample. The FRAP values of ascorbic acid and BHT were obtained by the same procedure.
Total phenolic content assay
The total phenolic content in the herbal extracts was measured by the Folin-Ciocalteu method, following the procedure of CitationSingleton et al. (1999). In brief, phenolic groups are oxidized by phosphomolybdic and phosphotungstic acids in Folin-Ciocalteu reagent, forming a green-blue complex detectable at 750 nm. In the test, 200 μ L of each extract solution of an appropriate concentration was mixed with 1 mL Folin-Ciocalteu reagent (1:10 diluted with H2O) and 800 μ L Na2CO3 (75.05 g/L). The absorbance at 750 nm was measured after 2 h reaction at room temperature. The aqueous gallic acid solution was freshly prepared in a series of concentrations (10∼ 80 μ g/mL) and tested in parallel to establish the standard curve. The total phenol content of each herbal extract was calculated as gallic acid equivalents.
Statistics
All data are expressed as means ± standard deviation (SD). Where applicable, analysis of variance was used for statistical evaluation of significant differences among the multiple groups. Differences were considered significant when p < 0.05. Correlation analyses were performed by Spearman's rank correlation analysis. All statistical analysis tests were performed by GraphPad Prism 4.02 for Windows (GraphPad Software, San Diego CA, USA).
Results and Discussion
Free radical scavenging capacity
In the DPPH assay, the percentage free radical scavenging capacity (SR%) of the extracts of different solvent fractions of the 40 kinds of Chinese medicinal herbs were assayed at a concentration of 7.5 mg/mL and the results are shown in . The SR% values of the fraction extracts ranged from undetectable for the water extract of Poria to 96.83% for the methanol extract of Radix rehmanniae. All of the four fraction extracts of Radix et rhizoma rhei, Cortex moutan, Rhizoma polygoni cuspidati, and Herba agrimoniae possessed high radical scavenging activity. Except for the petroleum ether fraction extract, the extracts of Herba taxilli, Herba epimedii, Fructus crataegi, and Radix sanguisorbae also exhibited a high radical scavenging rate. The SR% values of all four fraction extracts of Poria, Radix morindae officinalis, Semen persicae, and Rhizoma polygonati odorati showed weak radical scavenging capacity. In the total 146 fraction extracts assayed, 23.29% (34 samples) exhibited a SR% value greater than 85%. Half of these were extracts of methanol fractions. Most of the petroleum ether fraction extracts showed poor radical scavenging capacity, with 55.56% of these fraction extracts showing a SR% value < 10%. The substances of high radical scavenging and antioxidant activity, such as flavonoids, anthocyanins, carotenoids, vitamins, and other phenolic substances, are generally extracted into chloroform, methanol, and water, and exist less in petroleum ether (CitationWang, 1986), which is consistent with the results observed here.
In general, the SR% value of samples is of good concentration-dependent characteristic in the range from about 15% to 75% but does not increase proportionally with the concentration beyond this range especially in the case of high percentage free radical scavenging capacity. Therefore, in order to make a further exact evaluation of the antioxidant activity of the fraction extracts of high SR% at 7.5 mg/mL, the radical scavenging efficiency (SE) of the fraction extracts with a SR% greater than 50% was measured and is summarized in . When expressed as the SE value, the antioxidant power of some fraction extracts was not consistent with that presented as the SR% value at 7.5 mg/mL. The SE values changed between 13,698.63 and 128.89. The methanol fraction extract of Cortex cinnamomi showed the highest SE value of 13,698.63, which was higher than that of the two reference antioxidants, ascorbic acid and BHT. The methanol fraction extracts of Rhizoma polygoni cuspidati, Radix sanguisorbae, Semen arecae, Cortex moutan, and Herba taxilli also showed higher radical scavenging efficiency than did BHT.
Table 2 The free radical scavenging efficiency (SE) of the Chinese medicinal herbs.
Ferric reducing/antioxidant power
In the FRAP assay, the total antioxidant power of each fraction extract was calculated as the FRAP value of the fraction extract in μ mol/g. Some petroleum ether and chloroform–ethyl acetate fraction extracts were not suitable for assay, due to the poor solubility of these extracts in the aqueous test medium (). The water fraction extract of Radix sanguisorbae showed the strongest antioxidant power, with a FRAP value of 5202 μ mol/g. The lowest FRAP value (< 3 μ mol/g) was observed with the methanol fraction extract of Radix morindae officinalis. As compared with the FRAP value of the reference antioxidants, ascorbic acid exerted a stronger antioxidant activity than did all the other fraction extracts assayed. However, 19% of the fraction extracts exhibited a higher FRAP value than did BHT, and greater than 50% of these were methanol fraction extracts. Except for the petroleum ether fraction extract, all the solvent extracts of Radix sanguisorbae showed a higher FRAP value than did BHT. In addition, the methanol and water fraction extracts of Herba agrimoniae, Cortex cinnamomi, Herba epimedii, and Herba taxilli and the chloroform–ethyl acetate and methanol fraction extracts of Radix scutellariae were found to display better antioxidant activity than did BHT. The comparison of the antioxidant power among the extracts of different solvent fractions indicated that most of the methanol extracts showed the highest or second highest FRAP value in the four fractions of extracts of the medicinal herbs. The other three fractions demonstrated very different anti-oxidation capacity and there was no obvious rule.
Table 3 The FRAP value of the petroleum ether, chloroform-ethyl acetate, methanol and water fractions of the Chinese medicinal herbs.
Total phenol content
The total phenol content of the studied herbal fraction extracts ranged from undetectable to 587.92 mg of gallic acid equivalents/gram of extract (). Similar to the phenomenon occurring in FRAP assay, some petroleum ether and chloroform–ethyl acetate fraction extracts were not suitable for assay, due to their poor solubility in the test medium. Most of the methanol fraction extracts of the medicinal herbs showed higher total phenol content than did the other three fraction extracts. The highest total phenol content was observed with the methanol fraction extract of Semen arecae. The methanol fraction extract of Cortex cinnamomi and Herba taxilli contained the second and third highest total phenol content. The total phenol content in the petroleum ether fraction extract of Semen persicae, the chloroform-ethyl acetate fraction extract of Semen arecae, and the methanol fraction extract of Radix morindae officinalis were found to be the lowest three. Additionally, all fraction extracts of Radix ophiopogonis, Radix morindae officinalis, Semen persicae, and Rhizoma polygonati odorati contained very small amount of phenol substances. The extracts of some solvent fractions of Radix sanguisorbae, Rhizoma polygoni cuspidati, Herba agrimonia, Radix et rhizoma rhei, Cortex cinnamomi, Herba epimedii, and Herba taxilli exhibited fairly high total phenol content. From the general concept that the more phenol substances the sample contains, the higher antioxidant activity it shows, the total phenol content of these extracts basically matched their antioxidant power observed in the DPPH and FRAP assays.
Table 4 The total phenolics content in the petroleum ether, chloroform–ethyl acetate, methanol and water fractions of the Chinese medicinal herbs.
Comparative study between methods
Because of the complicated composition of the medicinal herbs and kinds of reaction mechanisms of the antioxidant assays, it is not reasonable to assess the anti-oxidation potency of samples by just one method (CitationKoleva et al., 2002). The DPPH assay and FRAP assay measure antioxidant power using different approaches. In this study, we found a significant positive correlation between the results obtained with the two methods (FRAP value vs. SE value, r = 0.8795, p < 0.001; n = 49).
Correlation between total phenolic content and antioxidant activity
Phenolic substances are one of the main kinds of antioxidants widely contained in natural plants, teas, and Chinese medicinal herbs (CitationLarson, 1988; CitationBenzie et al., 1999; CitationSun et al., 1999). In this study, most of the fraction extracts of powerful antioxidant activity contained higher phenolic content as well. As far as the free radical scavenging efficiency (SE) was concerned, the antioxidant activity of the fraction extracts analyzed was found to have a strong positive correlation with their total phenolic content (r = 0.7354, p < 0.001, n = 47). The FRAP value also showed a strong positive correlation with the total phenolic content (r = 0.9441, p < 0.001, n = 112). The results indicated that the phenolic substances played an important role in the antioxidant activity of the assayed fraction extracts from Chinese medicinal herbs.
In conclusion, the current study analyzed 40 commonly used Chinese medicinal herbs for their antioxidant capacity in a rapid and reproducible way. A database related to their antioxidant power was built. Results showed that the radical scavenging capacity and antioxidant power of the herbal extracts varied from very weak to very strong, with strongest activity in methanol and water extracts rather than in the less polar extracts, and that antioxidant power correlated significantly with the phenolic content. The DPPH and FRAP results correlated significantly, although the values were not the same. These data will be helpful in the search for herbs of strong antioxidant potency as possible candidates for further work on the characterization of specific antioxidant components and evaluation of their therapeutic significance in prevention of diseases induced by oxidative stress.
Acknowledgement
We are grateful for the technical support and assistance provided by Dr. S. B. Chen and Dr. H. L. Song (Institute of Modern Chinese Medicine, Institute of Materia Medica, The Hong Kong Polytechnic University, Shenzhen, China) and the technicians (the Open Lab of Chirotechnology, Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hong Kong, China). This work was supported by a grant from the Shenzhen Virttual University Park, Shenzhen, China.
References
- IFF Benzie, and JJ Strain. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem 239:70–76.
- IFF Benzie, and JJ Strain. (1999). Ferric reducing/ antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299:15–27.
- G Block, B Patterson, and A Subar. (1992). Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr Cancer 18:1–29.
- V Bondet, W Brand-Williams, and C Berset. (1997). Kinetics and mechanisms of antioxidant activity using the DPPHÿ free radical method. Lebensmittel-Wissenschaft und-Technologie 30:609–615.
- M Carini, G Aldini, G Rossoni, P Morazzoni, and RM Facino. (2001). Complexation of Ginkgo biloba extract with phosphatidylcholine improves cardioprotective activity and increases the plasma antioxidant capacity in the rat. Planta Med 67:326–330.
- CW Choi, SC Kim, SS Hwang, BK Choi, HJ Ahn, MY Lee, SH Park, and SK Kim. (2002). Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Science (Shannon, Ireland) 163:1161–1168.
- C Coudray, MJ Richard, and AE Favier. Determination of primary and secondary lipid peroxidation products: Plasma lipid hydroperoxides and thiobarbituric acid reactive substancesPierreedis, Analysis of Free Radicals in Biological SystemAE Favier, J Cadet, B Kalyanaraman, and MJ L Fontecave. Birkhauser Verlag, Berlin, (1995)185–200.
- B Fauconneau, P Waffo-Teguo, F Huguet, L Barrier, A Decendit, and JM Merillon. (1997). Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro tests. Life Sci 61:2103–2110.
- V Fogliano, V Verde, G Randazzo, and A Ritieni. (1999). Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J Agric Food Chem 47:1035–1040.
- A Ghiselli, M Serafini, G Maiani, E Azzini, and A Ferro-Luzzi. (1995). A fluorescence-based method for measuring total plasma antioxidant capability. Free Radic Biol Med 18:29–36.
- R Guillén-Sans, and M Guzmán-Chozas. (1998). The thiobarbituric acid (TBA) reaction in foods: A review. Crit Rev Food Sci Nutr 38:315–330.
- JMC Gutteridge, and B Halliwell. Antioxidants in Nutrition, Health, and Disease. Oxford University Press, New York, (1994)91.
- II Koleva, TA van Beek, JPH Linssen, A de Groot, and LN Evstatieva. (2002). Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem Anal 13:8–17.
- RA Larson. (1988). The antioxidants of higher plants. Phytochemistry 27:969–978.
- ZL Liu. (2001). Microenvironmental effects and synergistic effects of bio-antioxidants. Chin J Org Chem 21:884–889.
- NJ Miller, J Sampson, LP Candeias, PM Bramley, and CA Rice-Evans. (1996). Antioxidant activities of carotenes and xanthophylls. FEBS Lett 384:240–242.
- I Parejo, F Viladomat, J Bastida, A Rosas-Romero, N Flerlage, J Burillo, and C Codina. (2002). Comparison between the radical scavenging activity and antioxidant activity of six distilled and nondistilled Mediterranean herbs and aromatic plants. J Agric Food Chem 50:6882–6890.
- J Prakash, SK Gupta, V Kochupillai, N Singh, YK Gupta, and S Joshi. (2001). Chemopreventive activity of Withania somnifera in experimentally induced fibrosarcoma tumours in Swiss albino mice. Phytother Res 15:240–244.
- R Re, N Pellegrini, A Proteggente, A Pannala, M Yang, and C Rice-Evans. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237.
- M Saleem, A Alam, and S Sultana. (2001). Asafoetida inhibits early events of carcinogenesis: A chemopreventive study. Life Sci 68:1913–1921.
- K Schlesier, M Harwat, V Böhm, and R Bitsch. (2002). Assessment of antioxidant activity by using different in vitro methods. Free Radic Res 36:177–187.
- M Sharma, K Kishore, SK Gupta, S Joshi, and DS Arya. (2001). Cardioprotective potential of Ocimum sanctum in isoproterenol induced myocardial infarction in rats. Mol Cell Biochem 225:75–83.
- VL Singleton, R Orthofer, and RM Lamuela-Raventós. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299:152–178.
- PJK Steger, and SF Mühlebach. (1997). In vitro oxidation of IV lipid emulsions in different all-in-one admixture bags assessed by an iodometric assay and gas-liquid chromatography. Nutrition 13:133–140.
- CP Sun, JZ Zhang, and SJ Duan. Zi You Ji Sheng Wu Xue Dao Run. The Publishing House of Chinese Science and Technology University, Hefei, (1999).
- BP Vohra, SP Sharma, VK Kansal, and SK Gupta. (2001). Effect of Maharishi Amrit Kalash an ayurvedic herbal mixture on lipid peroxidation and neuronal lipofuscin accumulation in ageing guinea pig brain. Indian J Exp Biol 39:355–359.
- XK Wang. Medicinal Chemistry of Natural Products. The People's Medical Publishing House, Beijing, (1986)34.
- DDM Wayner, GW Burton, KU Ingold, and S Locke. (1985). Quantitative measurement of the total, peroxyl radical-trapping antioxidant capability of human blood plasma by controlled peroxidation. FEBS Lett 187:33–37.
- LP Yan, SW Chan, ASC Chan, SL Chen, XJ Ma, and HX Xu. (2006). Puerarin decreases serum total cholesterol and enhances thoracic aorta endothelial nitric oxide synthase expression in diet-induced hypercholesterolemic rats. Life Sci 79:324–330.
- HQ Zhang, and WX Yu. Zhong Hua Kang Shuai Lao Yi Yao Xue. Ci Xue Publishing House, Beijing, (2000)1–2.