Abstract
This study was carried out to elucidate the therapeutic efficacy of a new antimalarial drug, artemether, in adriamycin-induced nephropathy. To induce experimental nephrosis, adriamycin was given once by a single intravenous injection through the tail vein. Six days after adriamycin injection, therapeutic protocol was developed by intramuscular (i.m.) administration of 5 mg/kg artemether (ART). The total of i.m. injections were 14, in which five injections were made every day and nine injections were carried out at regular 48-h intervals. The therapeutic protocol was terminated on day 28, and animals were sacrificed on day 49. Our results showed that treatment with ART caused a significant reduction in the level of proteinuria (118 ± 15 vs. 48 ± 21 mg/24 h), urine urea (723 ± 226 vs. 414 ± 185 mg/dL), and urine sodium (38 ± 8 vs. 28 ± 6 mEq/L) compared with nontreated controls. In addition, a decrease in serum cholesterol (250 ± 58 vs. 163 ± 58 mg/dL) and creatinine (1.4 ± 0.2 vs. 0.9 ± 0.2 mg/dL) and an increase in the level of serum albumin (3.3 ± 0.5 vs. 5.6 ± 0.5 g/dL) were significant in the group treated with ART compared with the patient group. Moreover, treatment with ART significantly reduced glomerular PMN and mononuclear cells infiltration, hypercellularity, karyorrhexis, wire loops and hydropic change in the capillary network within the renal cortex, as well as decreased hyalin casts. On the other hand, healthy controls receiving ART showed a significant decrease in amounts of serum triglyceride (174 ± 55 vs. 88 ± 27mg/dL), urine urea (720 ± 113 vs. 511 ± 211 mg/dL), urine sodium (45 ± 5 vs. 26 ± 6 mEq/L), and potassium (71 ± 14 vs. 53 ± 6 mEq/L) compared with the normal group, where significant reduction of urinary excretion of sodium and urea must be considered as a side effect of ART therapy. These data suggest that artemether therapy can ameliorate proteinuria and suppress the progression of glomerular lesions in an experimental model of nephrosis, and that it may be recommended as a lipid-lowering drug.
Introduction
Artemisinin, a sesquiterpene lactone from Artemisia annua L. (“qinghao,” sweet wormwood), has raised considerable attention in past years (CitationEfferth, 2005). The genus Artemisia L. belongs to the family Composite. More than 350 Artemisia species are known, many of which have been used in traditional folk medicines for various applications (cough, blood circulation, diuresis, hypertension, allergy, parasites, etc.). Sesquiterpene lactones, flavonoids, coumarins, acetylenes, and sterols have been isolated from Artemisia species, some of which reveal antimalarial, antiviral, antitumor, antipyretic, anticoagulant, antispasmodic, and other effects (CitationTan et al., 1998; CitationEfferth et al., 2002a,b; CitationHaynes et al., 2005; CitationMahmoud & Botros, 2005; CitationVan der Meersch, 2005). The plant has been used in China for more than two millenia. Its first description dates back to the third century bc. Ge Hong (ad 281–340) recommended tea-brewed leaves to treat fever and chills in his “Handbook of Prescriptions for Emergency Treatment.” The “Compendium of Materia Medica” published by Li Shizen in 1596 cited Ge Hong's prescription. The fact that qinghao tea has withstood the centuries may be taken as a clue for the usefulness and activity of this prescription of traditional Chinese medicine (TCM) (CitationEfferth, 2005).
A program for the discovery of new antimalarial drugs from TCM launched by the Chinese government led in 1972 to the identification of artemisinin (“qinghaosu”), the active principle of A. annua L. (CitationKlayman, 1985; CitationButler & Wu, 1992). Because of the low solubility of artemisinin in oil and water, several semisynthetic derivatives have been developed, including artemether, arteether, artesunate, and others. The attractiveness of the artemisinin class of antimalarials is due to their activity against multidrug-resistant Plasmodium falciparum and Plasmodium vivax strains (CitationPrice et al., 1998; CitationGuthmann et al., 2006; CitationHaynes, 2006). Another salient feature is the lack of severe side effects in malaria patients (CitationRibeiro & Olliaro, 1998). Several million patients have been treated with these compounds during the past three decades (CitationDe Vries & Dien, 1996; CitationAceng et al., 2005; CitationFalade et al., 2005; CitationShen & Thiero, 2005; CitationSutherland et al., 2005; CitationMakanga et al., 2006; CitationMeremikwu et al., 2006; CitationOmari et al., 2006).
The antimalarial artemisinin derivatives also reveal antiangiogenic effect, as well as remarkable antineoplastic activity (CitationEfferth et al., 2002b; CitationWartenberg et al., 2003; CitationHe & Liu, 2004). They are able to inhibit tumor necrosis factor (TNF)-α mRNA expression (CitationKwiatkowski & Bate, 1995). Artemisinin and its derivatives also inhibit growth of many transformed cell lines. Their growth-inhibitory activity correlated with the induction of apoptosis and prevention of tumor angiogenesis, whereas apoptosis was not observed in normal endothelial cells (CitationWartenberg et al., 2003; CitationWu et al., 2004). These drugs inhibit angiogenesis in a dose-dependent manner. They markedly reduce vascular endothelial growth factor (VEGF) binding to its receptors and diminish the expression levels of two major VEGF receptors, Flt-1 and KDR/flk-1, on the surface of human vein endothelial cells (CitationChen et al., 2003). In addition, it has been shown that they can suppress IL-2 production (CitationSun, 1991), as well as inhibit inducible nitric oxide synthase and NF-kB activation (CitationAldieri et al., 2003), mechanisms by which rheumatoid arthritis (RA) could be ameliorated.
The aim of the current research is to assess the therapeutic potency of artemether in an experimental model of nephrosis and whether this drug could be recommended for treatment of glomerular lesions.
Materials and Methods
Animals
A total of 40 female Sprague-Dawley rats weighing 180 ± 20 g were obtained from Razi Institute and housed in our animal facility (temperature, 20–22°C; humidity, 55–56%; 12-h dark cycle; unlimited access to food and water) for at least 1 week before the experiment. The rats were divided at random into four groups: N, normal group (n = 8); P, patient group (n = 11); At, patient group treated with artemether (n = 11); E, experimental healthy control receiving artemether (n = 8). Experimental procedures were performed in accordance with the recommendations and policies of the Iran Pasteur Institute for the protection of animals used for experimental and other scientific purposes.
Experimental protocol and treatment
To induce nephrotic syndrome, adriamycin (Adriablastina; Farmitalia, Milan, Italy) was given once by a single intravenous injection (7.5 mg/kg) through the tail vein. Six days after adriamycin injection, a therapeutic protocol was developed by intramuscular (i.m.) administration of artemether (5 mg/kg) to group At. The total of i.m. injections were 14, in which five injections were made every day and nine injections were carried out at regular 48-h intervals. The therapeutic protocol was terminated on day 28, and animals were sacrificed on day 49. Normal group (N) received nothing, and group (E) was experimental healthy control receiving artemether.
Assessment of kidney function
Measurement of proteinuria was done during the eight stages. With start of treatment, urine was collected before the i.m. injections of artemether. The urine protein was measured using precipitation by trichloroacetic acid. The creatinine clearance was calculated by measuring creatinine concentration in urine and plasma by the alkaline picrate method (CitationBousnes & Taussky, 1945). Sodium and potassium concentrations were determined by flame photometry. Urine and blood urea nitrogen (BUN) were assessed by the Veniamin method (CitationVeniamin & Vakirtzi-Lemonias, 1970). Serum albumin was measured using the procedure of Bromcresol Green (CitationDoumas et al., 1971).
Evaluation of serum lipid levels
Serum triglyceride and cholesterol were determined by routine laboratory tests on the day of sacrifice.
Renal histopathologic studies
Kidney specimens were processed using light microscopy. Renal tissues were fixed by immersion in 10% buffered formalin, embedded in paraffin, and 4-μ m sections were stained with hematoxylin-eosin and periodic acid–Schiff. The severity and extent of glomerular lesions were blindly evaluated for seven parameters: hypercellularity, glomerular infiltration of PMN and mononuclear cells, karyorrhexis, wire loops, hyalin casts, and cellular swelling (hydropic change) in capillary network within the renal cortex. They were evaluated by a semiquantitative method of renal histology using a grading scale of 0 to 3 (0, negative; 1, mild; 2, moderate; 3, severe).
Toxicologic studies
To evaluate the side effects of artemether in normal rats, the experimental group (E) was studied. These healthy controls received only intramuscular injections of artemether at one dose (5 mg/kg). In this group, the number and interval of injections was 12 and 48 h, respectively. Two days after the last injection, toxicologic studies were done as follows:
Assessment of kidney function was performed based on the measurements of urinary protein excretion, serum albumin, urine and plasma creatinine, urine and BUN, and sodium and potassium concentrations in urine and plasma.
Evaluation of serum lipid levels was carried out by determining serum triglyceride and cholesterol concentrations.
Renal tissues were assessed using light microscopy. Glomerular lesions were graded on a scale of 0 to 3 (0, negative; 1, mild; 2, moderate; 3, marked) according to seven parameters: glomerular infiltration of PMN and mononuclear cells, hypercellularity, karyorrhexis, wire loops, cellular swelling (hydropic change), and the presence of hyalin casts.
Statistical analysis
Data were expressed as means ± SD and ± SEM. Statistical analysis was performed using the Student's t-test for parametric data and Mann-Whitney test for nonparametric data. p values < 0.05 were considered significant.
Results
Effect of artemether on renal function
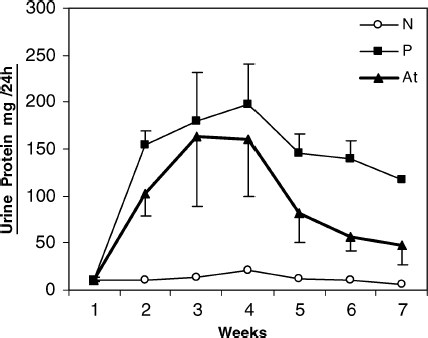
The changes of the mean levels of urinary protein excretion between N, P, and At are shown in . This experiment showed that i.m. administration of artemether (5 mg/kg) could exert therapeutic effects on adriamycin-induced nephropathy. For exact evaluation of artemether (ART) effects, the experiment was terminated 20 days after the last ART injection. The urinary protein excretion was significantly less in ART-treated animals compared with that of nontreated controls (P < 0.05).
Figure 1 Time course of the mean values of proteinuria in different groups of N, P, At in experimental nephrosis. N, normal rats (n = 8); P, patient (nontreated) rats that show increase of proteinuria levels after day 6 (n = 11); At, treated group by intramuscular (i.m.) injection of artemether, 5 mg/kg (n = 11). Note: A single intravascular injection of adriamycin (7.5 mg/kg body weight) induced a severe nephrotic syndrome. Onset of i.m. administration of artemether was day 6 (after development of disease). There were significant differences between nontreated (P) and artemether-treated rats (At). p < .05 was considered to be significant.
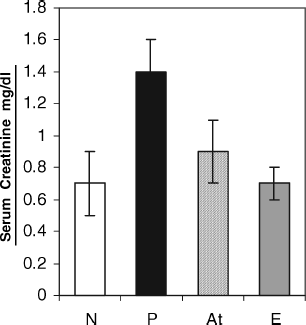
shows the effect of ART on urine parameters in groups N, P, At, and E. In , comparison of antiproteinuric effect of ART is made between various groups at the end of the experiment (day 49). Here, reduction of proteinuria in At (48 ± 21) versus P (118 ± 15 mg/24 h) was significant. demonstrates the concentration of urine sodium in different groups, in which At (28 ± 6) vs. P (38 ± 8 mEq/L) was significant. In , the amounts of urine urea nitrogen have been compared between the groups (N, P, At, and E). This figure shows that the difference between treated rats (At) and nontreated animals (P) is significant (414 ± 185 vs. 723 ± 226 mg/dL) “P < 0.05”. In , the comparison of serum creatinine concentration among various groups shows that there is a significant difference between At vs. P (0.9 ± 0.2 vs. 1.4 ± 0.2 mg/dL) (p < 0.05).
Figure 2 Effect of artemether on urine parameters in groups: N, normal (8); P, patient (11); At, treated rats with artemether (11); and E, experimental healthy control receiving artemether. Each bar represents mean ± SD. p < .05 vs. nontreated. (A) Comparison of antiproteinuric effect of artemether between groups N, P, At, and E at the end of experiment (day 49); At vs. P was significant. (B) Concentration of urine sodium in groups N, P, At, and E; At vs. P was significant. (C) Amounts of urine urea nitrogen in groups N, P, At, and E; At vs. P was significant.
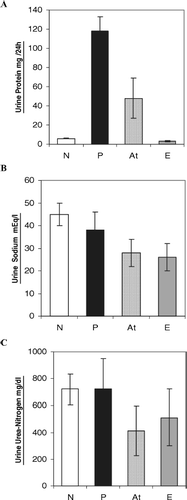
Figure 3 Effect of artemether on serum creatinine in different groups: N, normal (8); P, patient (11); At, treated with artemther (11); E, experimental healthy control receiving artemther (8). Each bar represents the mean ± SD. Treatment with artemether showed a significant reduction in creatinine level compared with the patient group (p < .05).
Effect of artemether on serum lipid levels
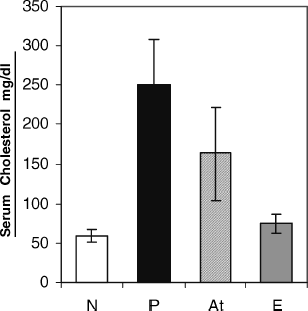
Serum cholesterol and triglyceride levels were significantly elevated (p at least < 0.05) in nephrotic rats (P) when compared with the healthy controls (N) at the end of experiment (60 ± 8 vs. 250 ± 58 mg/dL and 174 ± 55 vs. 427 ± 62 mg/dL, respectively). Intramuscular injections of ART solution to patient rats significantly reduces serum cholesterol in nephrotic animals (250 ± 58 vs. 163 ± 58 mg/dL) ().
Figure 4 Effect of artemether on serum cholesterol level in groups: N, normal (8); P, patient (11); At, Treated with artemther (11); E, experimental healthy control receiving artemther (8). Each bar represents the mean ± SD. Decrease in cholesterol concentration in group At vs. P was significant (p < .05).
Histopathologic findings
Light microscopic examination of renal tissue revealed the severity of hypercellularity, glomerular infiltration of PMN, karyorrhexis, wire loops, hydropic change in capillary network within the renal cortex, and existence of tubular casts in the various groups. The animals treated with ART showed a significant reduction in glomerular changes relative to nontreated controls ( and ).
Figure 5 Representative histopathologic slides of kidney specimens of a healthy rat (N), a control rat with adriamycin-induced nephropathy (P), a nephrotic animal treated with artemether (At), and healthy controls receiving ART (E). The rats that had been treated with artemether show significantly fewer signs of glomerular destruction (hematoxyline-eosin; original magnification × 40).
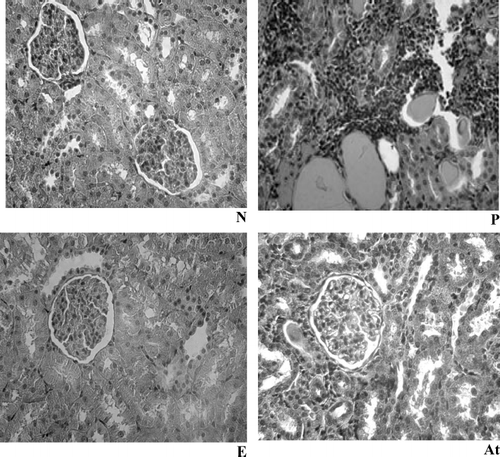
Table 1 Light microscopic findings of kidney histologic lesions in various groups.
Toxicologic findings
and show a comparison between four groups (N, P, At, and E) in serum and urine parameters. summarizes the data concerning the effect of ART administration on serum parameters. In this examination, with the exception of triglyceride, there were no significant differences in the levels of serum determinants between the normal group (N) and healthy controls receiving ART (E), whereas, a decrease in triglyceride was significant (174 ± 55 vs. 88 ± 27 mg/dL) (p < 0.05). represents the data concerning the effect of ART administration on urine parameters. Data analysis showed a significant difference in amounts of urine sodium (45 ± 5 vs. 26 ± 6 mEq/L), potassium (71 ± 14 vs. 53 ± 6 mEq/l), and urine urea (720 ± 113 vs. 511 ± 211 mg/dL), between the normal group (N) versus E (p < 0.05). Histologic examinations of kidney specimens obtained from N and E groups showed no glomerular changes in group E compared with group N ().
Table 2 Effect of artemether on serum determinants.
Table 3 Effect of artemether on urine determinants.
Discussion
This study was developed in an attempt to test the therapeutic effect of artemether as a new antimalarial drug in experimental nephrotic syndrome. This syndrome is characterized by massive proteinuria, edema, hyperlipidemia, and hypoalbuminemia (CitationCunard & Kelly, 2003). Adriamycin-induced nephropathy serves as an experimental model for minimal change nephropathy, and focal and segmental glomerulosclerosis (CitationRemuzzi et al., 1985). Oxidative stress and superoxide, hydrogen peroxide, and, consequently, hydroxyl radical production are suggested to play a role in its pathogenesis (CitationOkasora et al., 1992; CitationZima et al., 1998), and cytokines released by circulating blood mononuclear cells and/or resident renal cells could alter the permeability of the glomerular capillary wall (CitationBustos et al., 1995). Accumulated evidence indicates increased expression of IL-2 mRNA, a significant increase in TNF-α and nitric oxide (NO) production levels, as well as a significant increase in plasma and urinary VEGF levels in human and experimental model of nephrotic syndrome, suggesting that IL-2, TNF-α, NO, and VEGF, at least in part, might be involved in the pathophysiology of this disease (CitationOzen et al., 2001; CitationZhu et al., 2001; CitationLama et al., 2002; CitationZachwieja et al., 2002, Citation2003; CitationKlahr & Morrissey, 2004; CitationShimoyama et al., 2004; CitationUsta et al., 2004; CitationDeepa & Varalakshmi, 2006; CitationWasilewska et al., 2006; CitationWasilewska & Zoch-Zwierz, 2006), whereas the antimalarial artemisinin derivatives markedly reduce VEGF binding to its receptors and diminish the expression levels of two major VEGF receptors, Flt-1 and KDR/flk-1, on the surface of human vein endothelial cells (CitationChen et al., 2003). In addition, it has been shown that they can suppress IL-2 production (CitationSun, 1991) and inhibit inducible nitric oxide synthase, and they are able to suppress (TNF-α mRNA expression (CitationKwiatkowski & Bate, 1995). Our findings show that artemether therapy not only is able to reduce proteinuria, but also it could significantly diminish urine urea and sodium compared with nontreated controls. In addition, decrease in serum creatinine and increase in the level of serum albumin were significant in the group treated with ART compared with the patient group. Moreover, our data in the current work revealed that treatment with ART significantly reduces serum cholesterol levels in the treated patient rats in experimental nephrosis. Nephrotic syndrome results in downregulation of LDL receptor expression, which, in turn, contributes to the pathogenesis of hypercholesterolemia and dysregulation of cholesterol synthesis and catabolism (CitationPesek-Diamond et al., 1992; CitationVaziri & Liang, 1996). Our results show that plasma cholesterol concentration in the nephrotic animals and normal group are in agreement with the studies of CitationVaziri and Liang (1996). Also, ART therapy not only did not exacerbate renal damage, but interestingly, administration of this drug could significantly diminish renal recruitment of leukocytes and reduce glomerular lesions in experimental model of nephrotic syndrome. On the other hand, healthy controls receiving ART showed a significant decrease in amounts of serum triglyceride, urine urea, and urine sodium and potassium compared with the normal group. Here, the significant reduction in amount of urine urea and urine sodium after ART therapy in the treated group and healthy controls receiving ART compared with the normal group may be an important factor for a further precision in administration of this drug.
In conclusion, a significant reduction in proteinuria, serum cholesterol, and serum creatinine, as well as suppression of glomerular lesions after artemether therapy suggests its therapeutic benefit in the treatment process of experimental nephrosis. But in long-term administration of this drug, reduction of urinary excretion of sodium and urine urea must be considered as a side effect of artesunate therapy.
References
- JR Aceng, JS Byarugaba, and JK Tumwine. (2005). Rectal artemether versus intravenous quinine for the treatment of cerebral malaria in children in Uganda: Randomized clinical trial. BMJ 330:317–318.
- E Aldieri, D Atragene, L Bergandi, C Riganti, C Costamagna, A Bosia, and D Ghigo. (2003). Artemisinin inhibits inducible nitric oxide synthase and nuclear factor NF-kB activation. FEBS Lett 552:141–144.
- RW Bousnes, and AA Taussky. (1945). The calorimetric determination of creatinine by the Jaffe reaction. J Biol Chem 158:581–591.
- C Bustos, S Gonzales-Cuadrado, M Ruiz-Ortega, C Gomes-Guerrero, E Gonzalez, JJ Plaza, and J Egido. (1995). Cyclosporin A (CsA) modulates the glomerular production of inflammatory mediators and proteoglycans in experimental nephrosis. Clin Exp Immunol 102:608–613.
- AR Butler, and YL Wu. (1992). Artemisinin (qinghaosu)—a new type of antimalarial drug. Chem Soc Rev 21:85–90.
- HH Chen, HJ Zhou, and X Fang. (2003). Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharmacol Res 48:231–236.
- R Cunard, and CJ Kelly. (2003). Immune-mediated renal disease. J Allergy Clin Immunol 111:637–643.
- PR Deepa, and P Varalakshmi. (2006). Influence of a low-molecular-weight heparin derivative on the nitric oxide levels and apoptotic DNA damage in adriamycin-induced cardiac and renal toxicity. Toxicology 217:176–183.
- PJ De Vries, and TK Dien. (1996). Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs 52:818–836.
- BT Doumas, WA Watson, and HG Biggs. (1971). Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chem Acta 31:87–88.
- T Efferth. (2005). Mechanistic perspectives for 1,2,4-trioxanes in anti-cancer therapy. Drug Resist Updat 8:85–87.
- T Efferth, M Marschall, X Wang, SM Huong, I Hauber, and A Olbrich. (2002a). Antiviral activity of artesunate towards wild-type, recombinant, and ganciclovir-resistant humancytomegaloviruses. J Mol Med 80:233–242.
- T Efferth, A Olbrich, and R Bauer. (2002b). mRNA expression profiles for the response of human tumor cell lines to the antimalarial drugs artesunate, arteether, and artemether. Biochem Pharmacol 64:617–623.
- C Falade, M Makanga, Z Premji, CE Ortmann, M Stockmeyer, and PI de Palacios. (2005). Efficacy and safety of artemether-lumefantrine (Coartem) tablets (six-dose regimen) in African infants and children with acute, uncomplicated falciparum malaria. Trans R Soc Trop Med Hyg 99:459–467.
- JP Guthmann, S Cohuet, C Rigutto, F Fortes, N Saraiva, J Kiguli, J Kyomuhendo, M Francis, F Noel, M Mulemba, and S Balkan. (2006). High efficacy of two artemisinin-based combinations (artesunate + amodiaquine and artemether + lumefantrine) in Caala, Central Angola. Am J Trop Med Hyg 75:143–145.
- RK Haynes. (2006). From artemisinin to new artemisinin antimalarials: Biosynthesis, extraction, old and new derivatives, stereochemistry and medicinal chemistry requirements. Curr Top Med Chem 6:509–537.
- RK Haynes, HW Chan, WY Ho, CK Ko, L Gerena, DE Kyle, W Peters, and BL Robinson. (2005). Convenient access both to highly antimalaria-active 10-arylaminoartemisinins, and to 10-alkyl ethers including artemether, arteether, and artelinate. Chembiochem 6:659–667.
- XL He, and Z Liu. (2004). Protection of artesunate on activation and injury of vascular endothelial cells induced by lipopolysaccharide. Zhongguo Zhong Xi Yi Jie He Za Zhi 24:1110–1113.
- S Klahr, and J Morrissey. (2004). l-Arginine as a therapeutic tool in kidney disease. Semin Nephrol 24:389–394.
- DL Klayman. (1985). Qinghaosu (artemisinin): An antimalarial drug from China. Science 228:1049–1055.
- D Kwiatkowski, and C Bate. (1995). Inhibition of tumour necrosis factor (TNF) production by antimalarial drugs used in cerebral malaria. Trans R Soc Trop Med Hyg 89:215–216.
- G Lama, I Luongo, G Tirino, A Borriello, C Carangio, and ME Salsano. (2002). T-lymphocyte populations and cytokines in childhood nephrotic syndrome. Am J Kidney Dis 39:958–965.
- MR Mahmoud, and SS Botros. (2005). Artemether as adjuvant therapy to praziquantel in murine Egyptian Schistosomiasis mansoni. J Parasitol 91:175–178.
- M Makanga, Z Premji, C Falade, J Karbwang, EA Mueller, K Andriano, P Hunt, and PI De Palacios. (2006). Efficacy and safety of the six-dose regimen of artemether-lumefantrine in pediatrics with uncomplicated Plasmodium falciparum malaria: A pooled analysis of individual patient data. Am J Trop Med Hyg 74:991–998.
- M Meremikwu, A Alaribe, R Ejemot, A Oyo-Ita, J Ekenjoku, C Nwachukwu, D Ordu, and E Ezedinachi. (2006). Artemether-lumefantrine versus artesunate plus amodiaquine for treating uncomplicated childhood malaria in Nigeria: Randomized controlled trial. Malaria J 5:44–45.
- T Okasora, T Takikawa, and Y Utsunomiya. (1992). Suppressive effect of superoxide dismutase on adriamycin nephropathy. Nephron 60:199–203.
- AA Omari, C Gamble, and P Garner. (2006). Artemether-lumefantrine (four-dose regimen) for treating uncomplicated falciparum malaria. Cochrane Database Syst Rev 2: CD005965
- S Ozen, Y Usta, I Sahin-Erdemli, D Orhan, B Gumusel, B Yang, Y Gursoy, O Tulunay, T Dalkara, A Bakkaloglu, and M El-Nahas. (2001). Association of nitric oxide production and apoptosis in a model of experimental nephropathy. Nephrol Dial Transplant 16:32–38.
- I Pesek-Diamond, G Ding, J Frye, and JR Diamond. (1992). Macrophages mediate adverse effects of cholesterol feeding in experimental nephrosis. Am J Physiol 263:776–783.
- R Price, M van Vugt, F Nosten, C Luxemburger, A Brockman, L Phaipun, T Chongsuphajaisiddhi, and N White. (1998). Artesunate versus artemether for the treatment of recrudescent multidrug-resistant falciparum malaria. Am J Trop Med Hyg 59:883–888.
- G Remuzzi, C Zoja, and A Remuzzi. (1985). Low-protein diet prevents glomerular damage in adriamycin-treated rats. Kidney Int 28:21–27.
- IR Ribeiro, and P Olliaro. (1998). Safety of artemisinin and its derivatives. A review of published and unpublished clinical trials. Med Trop 58:50–53.
- WJ Shen, and M Thiero. (2005). Effects of combined artemether and dihydroartemisinin in treatment of children with falciparum malaria. Zhonghua Er Ke Za Zhi 43:65–66.
- H Shimoyama, M Nakajima, H Naka, Y Maruhashi, H Akazawa, T Ueda, M Nishiguchi, Y Yamoto, H Kamitsuji, and A Yoshioka. (2004). Up-regulation of interleukin-2 mRNA in children with idiopathic nephrotic syndrome. Pediatr Nephrol 19:1115–1121.
- XZ Sun. (1991). Experimental study on the immunosuppressive effects of qinghaosu and its derivative. Zhong Xi Yi Jie He Za Zhi 11:37–38.
- CJ Sutherland, R Ord, S Dunyo, M Jawara, CJ Drakeley, N Alexander, R Coleman, M Pinder, G Walraven, and GA Targett. (2005). Reduction of malaria transmission to anopheles mosquitoes with a six-dose regimen of co-artemether. PloS Med 2:92–93.
- RX Tan, WF Zheng, and HQ Tang. (1998). Biologically active substances from the genus Artemisia. Planta Med 64:295–302.
- Y Usta, UB Ismailoglu, A Bakkaloglu, D Orhan, N Besbas, I Sahin-Erdemli, and S Ozen. (2004). Effects of pentoxifylline in adriamycin-induced renal disease in rats. Pediatr Nephrol 19:840–843.
- H Van der Meersch. (2005). Review of the use of artemisinin and its derivatives in the treatment of malaria. J Pharm Belg 60:23–29.
- ND Vaziri, and KH Liang. (1996). Down-regulation of hepatic LDL receptor expression in experimental nephrosis. Kidney Int 50:887–893.
- MP Veniamin, and C Vakirtzi-Lemonias. (1970). Chemical basis of the carbamidodiacetyl micromethod for estimation of urea, citrulline and carbamyl derivatives. Clin Chem 16:3–6.
- M Wartenberg, S Wolf, P Budde, F Grunheck, H Acker, J Hescheler, G Wartenberg, and H Sauer. (2003). The antimalaria agent artemisinin exerts antiangiogenic effects in mouse embryonic stem cell-derived embryoid bodies. Lab Invest 83:1647–1655.
- A Wasilewska, and W Zoch-Zwierz. (2006). Glucocorticoid receptor and vascular endothelial growth factor in nephrotic syndrome. Acta Paediatr 95:587–593.
- A Wasilewska, W Zoch-Zwierz, and E Tenderenda. (2006). Vascular endothelial growth factor in children with nephrotic syndrome treated with cyclosporine A. Acta Paediatr 95:291–296.
- GD Wu, HJ Zhou, and XH Wu. (2004). Apoptosis of human umbilical vein endothelial cells induced by artesunate. Vascul Pharmacol 41:205–212.
- J Zachwieja, W Bobkowski, A Dobrowolska-Zachwieja, M Lewandowska-Stachowiak, M Zaniew, and J Maciejewski. (2002). Intracellular cytokines of peripheral blood lymphocytes in nephrotic syndrome. Pediatr Nephrol 17:733–740.
- J Zachwieja, W Bobkowski, M Zaniew, D Runowski, M Lewandowska-Stachowiak, and J Maciejewski. (2003). Intracellular synthesis of cytokines as the index of function of peripheral blood lymphocytes and monocytes in children with first relapse of nephrotic syndrome. Pol Merkuriusz Lek 14:289–294.
- Z Zhu, Y Wang, H Wang, and A Deng. (2001). The study on the relationship between serum vascular endothelial growth factor and proteinuria in adriamycin-induced nephrotic rats. J Tongji Med Univ 21:301–303.
- T Zima, V Tesar, J Crkovska, A Stejskalova, J Platenik, J Teminova, K Nemecek, M Janebova, and S Stipek. (1998). ICRF-187 (dexrazoxan) protects from adriamycin-induced nephrotic syndrome in rats. Nephrol Dial Transplant 13:1975–1979.