Abstract
Seasonal variations of total phenolic, antioxidant activity, PNE (Plant Nutrient Elements), and fatty acids in fresh tea leaves grown in Turkey were studied. Fresh tea leaves sampled from Derepazari 7 clone [Camellia sinensis L. var. sinensis (Theaceae)] were analyzed and compared during the three commercial harvest seasons (May 15, July 15, and September 15) in both 2005 and 2006. The levels of total phenolics and antioxidant activity was higher at 2nd harvest time (62.88 μ g/mg and 89.27%). The seasonal variations of the individual fatty acids were significant (P < 0.05) between the three harvest seasons. The amount of N and P in tea leaves was the highest at 1st harvest; however K, Ca, Mg, S, and Mn were highest at 2nd harvest time. This study revealed that total phenolics could be used as quality descriptors for monitoring the seasonal variations in Turkey-grown tea leaves.
Introduction
Tea (Camellia sinensis L.) belongs to Theaceae family and is one of the most popular beverages in the world (CitationFernandez et al., 2002). China and India are major tea producers (941.000 and 831.000 tons) followed by Sri Lanka (308.000 tons), Kenya (295.000 tons) and Turkey (202.000 tons), respectively (Anon., 2005). The production of tea in Turkey began in the early years of the Republic along the Eastern Black Sea Region and most of the tea plantations are centered on the Rize city in the country (CitationMendilcioglu, 2000).
Tea is drunk in almost every country around the world and has reached a ceremonial status in many places both as a social and medicinal beverage. Since 3000 B.C., traditional Chinese medicine has recommended green tea for headaches, body aches and pains, digestion, enhancement of immune system, detoxification, as an energizer, and to prolong life (CitationXie et al., 1998). The health benefits of tea are confirmed and the therapeutic value of tea for the prevention and treatment of many diseases has become more and more commonly known (Zaveri et al., 2002).
Total phenolic content present in young tea shoots (also referred to as fresh green leaves, fresh tea shoots, or flushes) are known to be one of the main factors in determining the quality of the resulting tea drink (CitationHara, 1995). The content of total phenolics in tea shoots grown in Kenya has been found to correlate significantly with Kenyan plain tea quality parameters and clones with low total phenolic content produced low quality black teas (CitationObanda et al., 1992, Citation1997). In other words, the quality of black tea is dependent in the first instance on the chemical composition, in particular, the flavanols of the harvested shoots, and in the second instance by the way in which they are handled, processed, and stored (CitationSerafini et al., 1996). Tea leaves also have high antioxidant activity (CitationHara, 1995). Dietary intake of antioxidant compounds is important for health (CitationDuh et al., 1999). Although there are some synthetic antioxidants such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) which is commonly used in processed foods, it has been reported that these compounds have some side effects (CitationIto et al., 1983). Another healthy function of some plants is their essential fatty acid composition, which humans cannot synthesize, but must obtain through diet. Essential fatty acids regulate numerous body functions including blood pressure, blood viscosity, immune and inflammatory responses (CitationPawlosky et al., 1996; CitationSimopoulos & Salem, 1996). Tea also contains minerals and trace elements such as K, Mn, Cr, Ni, and Zn which are essential to human health. The regular consumption of tea may contribute to the daily dietary requirements of several elements and tea could be an important source of manganese, and the large amount of potassium in comparison with sodium that could be beneficial for hypertensive patients (CitationXie et al., 1998; CitationFernandez et al., 2002).
There were studies related to chemical composition of tea leaves (CitationXie et al., 1998; CitationFerrara et al., 2001; CitationFernandez et al., 2002). However, the variation of chemical content present in tea leaves in different harvest time in Turkey has not been studied so far. Moreover, to our knowledge, fatty acid contents of tea leaves have not been reported in literature.
Materials and Methods
Collection and preparation of tea leaf samples
Tea leaves were harvested from dominant clone Derepazari-7 belongs to Camellia sinensis var. sinensis from Rize city of Turkey at three commercial harvest times (May 15, July 15, and September 15) in both 2005 and 2006 year. Plant materials were further identified by senior taxonomists, Dr. Meryem Sengul, from Department of Botany, Ataturk University, Erzurum, Turkey. There were no statistical differences between the two years when the results were analyzed, therefore the data of the different years on total phenolics, antioxidant activity, fatty acids, and plant nutrient elements were pooled (this could be a consequence of similar growing and climatic conditions in both years where the plants grow). After harvest, the leaves were cleaned and cut into small pieces before being dried in a hot air-blowing oven at 45°C. All samples after drying were ground to a fine powder prior to extraction.
Determination of fatty acid content
Fatty acid composition was analyzed according to a previous method (Anon., 2000). Fatty acids were designated (e.g., 18:1 ω 9c) so that the figures represent, from left to right, the total number of carbon atoms (i.e., 18), the number of double bonds (i.e., 1), the position of the double bond from the ω end of the fatty acyl chain (i.e., ω 9) and the configuration of the double bond (i.e., c for cis).
Determination of antioxidant activity
The antioxidant activity of methanol extract of tea leaves was determined according to the β -carotene bleaching method described by CitationKaur and Kapoor (2002). Grounded sample (10 mg) was mixed with 10 ml methanol and stirred for 30 min on a magnetic stirrer. The suspension was filtered through Whatman No. l filter paper. Final solutions were used as stock solution for the antioxidant activity and phenolic analysis. Briefly, 4 ml of β -carotene solution (0.1 mg in 1 ml chloroform), 40 mg of linoleic acid and 400 mg of Tween 40 were transferred to a round-bottom flask. The mixture was then evaporated at 50°C by means of a rotary evaporator to remove chloroform. Then, 100 ml of oxygenated distilled water were added slowly to the residue and vigorously agitated to give a stable emulsion. Then, 800 μ l of extracts were added to 3 ml aliquots of β -carotene/linoleic acid emulsion. As soon as the emulsion was added to each tube, the zero time absorbance was measured at 470 nm using a spectrophotometer. The mixtures were incubated at 50°C for 90 min. The measurement was carried out at 15 min intervals for 90 min. Methanol were used as control. A blank, devoid of β -carotene, was prepared for background subtraction. BHA and BHT were used as a standard. All samples were assayed in duplicate. Degradation rate (DR) was calculated according to first order kinetics, using the following equation based on:
Where ln is natural log, a is the initial absorbance (470 nm) at time 0, b is the absorbance (470 nm) at 100 min and t is time. Antioxidant activity (AA) was expressed as percent of inhibition relative to the control, using the following formula:
Determination of total phenolics in tea leaves
The concentration of total phenolics in the methanol extract of tea leaves was determined by the Folin–Ciocalteau colorimetric method (CitationNaczk & Shahidi 1989). Briefly, 1 ml of the solution (contains 1 mg) extract in water was pipetted into a flask. Then 46 ml of distilled water and 1 ml of Folin-Ciocalteu's reagent was added and mixed thoroughly. The mixture was left to stand for 3 min and 3.0 ml of 2% sodium carbonate were added. After 120 min incubation at ambient temperature with shaking, the resulting absorbance was measured at 760 nm. Measurements were carried out in duplicate and the calibration curve was performed with gallic acid, and the results were expressed as μ g of gallic acid equivalents per miligram (μ g GAE/mg).
Determination of mineral elements
Total N was determined by the micro Kjeldahl method (CitationJames, 1995). In order to determine the mineral composition, samples were burned with a nitric acid and perchloric acid solution, on the hot plate, at 200°C. Then, the absorbance of the extract was measured by the Atomic Absorbance Spectrophotometer. The amounts of minerals were calculated with a standard curve of each element. Phosphorus content of the extract, however, was analyzed by determining the absorbance of the color yellow, obtained from the Barton reaction, using a spectrophotometer (Thermo, Nicolet 100, UV) at 680 nm wavelengths, and comparing the results to the Standard curve.
Statistical analysis
The experiment was a completely randomized design with four replications. Data were subjected to analysis of variance (ANOVA) and means were separated by Duncan multiple range test at P < 0.05 significant level.
Results and Discussion
Fatty acid composition of tea leaves
Fatty acid analysis has shown that tea leaves studied contained five major compounds and a much greater variation of fatty acids was found among different harvest times (). α -Linoleic acid was the dominant fatty acid (29.02%) in tea leaves at 1st harvest time followed by palmitic acid (25.26%) and nervonic acid (16.62%), respectively. In 2nd harvest time this figure was changed and nervonic acid determined as major fatty acid in tea leaves (24.75%) followed by tricosanoic acid (24.38%) and α -linoleic acid (19.82%), respectively. At 3rd harvest time α -linoleic acid again was major fatty acid in tea leaves (29.05%) followed by nervonic (23.28%) and tricosanoic (20.28) acid, respectively (). Total peak areas of the mentioned fatty acids in tea leaves were 100.00% at 1st harvest, 95.17% at 2nd harvest, and 100.00% at 3rd harvest time (). The fatty acid compounds contribute to the flavor of tea leaves, which has distinct taste rather than aroma. According to literature searched, there is no study on fatty acid content of tea leaves. Thus, our fatty acid results may be the first study to provide data that the tea leaves possess fatty acids.
Table 1. Fatty acid content (%) of tea leaves.
Antioxidant activity of tea leaves
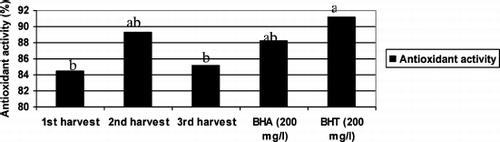
Antioxidant activity of tea leaves are given in . The differences among harvest times, BHA and BHT were found statistically important. Antioxidant activity of tea leaves were high and found to be 84.43% at 1st harvest and increased to 89.27% at 2nd harvest and decreased to 85.17% at 3rd harvest time. The antioxidant activity of BHA (200 mg/l) and BHT (200 mg/l) were 88.24 and 91.18%, respectively (). In previous studies conducted on different tea samples using different solvents, the antioxidant activity was found between 2–83% (CitationTurkmen et al., 2006). CitationJuliani and Simon (2002) reported that antioxidant activity of green tea 4–5 times higher than cinnamon (Ocimum basilicum L., Lamiaceae) and oregano (Origanum vulgare L., Labiatae). CitationTavazzi & Offord (2001) revealed that commonly consumed products such as tea, coffee, and cocoa have possessing significant antioxidant activity. The results for antioxidant activity clearly outline that tea leaves could be one of the richest sources of plants in terms of antioxidant activity. The great difference of tea leaves for antioxidant activity at different harvest time is supposed to the effect of change of ecological parameters. It was previously reported that, the composition of tea leaves varies with climate, variety, and age of the leaf (CitationLeung & Foster, 1996). Tea and its constituent catechins are best known for their antioxidant properties, which has led to their evaluation in a number of diseases associated with reactive oxygen species (ROS), such as cancer, cardiovascular, and neurodegenerative diseases. Several epidemiological studies as well as studies in animal models have shown that green tea can afford protection against various cancers such as those of the skin, breast, prostate and lung (CitationMukhtar & Ahmad, 2000; CitationYang et al., 2002).
Total phenolics in tea leaves
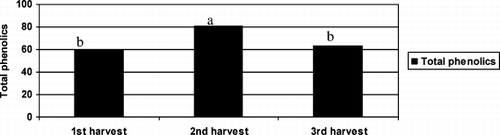
The total phenolic content of tea leaves are given in . Total phenolic content were 59.44 μ g/mg at 1st harvest and increased to 80.69 μ g/mg at 2nd harvest and decreased to 62.88 μ g/mg at 3rd harvest time (). The level of total phenolics has been found to positively correlate with the antioxidant activity in the tea leaves (R = 0.891). CitationRobertson (1992) previously reported that tea leaves are extremely rich in phenolic compounds which can constitute up to 300 mg/g of material. CitationJuliani and Simon (2002) are also reported that green tea leaves is very rich for total phenolics and total phenolic content of green tea leaves 4-5 times higher than cinnamon (Ocimum basilicum) and oregano (Origanum vulgare). They found strong relationships between antioxidant activity and total phenolics in tea leaves which support our findings. It has been shown that the biosynthesis of phenolic compounds can be effectively induced by sunlight (CitationHarbowy & Balentine 1997). That is why in shaded tea flushes the concentrations of total phenolics are much lower. On the basis of this information, the differences in total phenolic levels between fresh leaves harvested in cooler (May and September) and warmer months (July) in Turkey may not be just a temperature effect but also a day length and sunlight effect. The highest total phenolic levels in tea are important for public by reducing the risk of atherosclerosis and coronary heart disease, which can be caused by oxidation of low-density lipoproteins (CitationShahidi & Wanasundara, 1992).
Mineral elements in gren tea leaves
The mineral contents of tea leaves at different harvest times are shown in . Differences among the different harvest times were observed based on the mineral compositions (). The amount of N and P in tea leaves was the highest at 1st harvest; however K, Ca, Mg, S and Mn were highest at 2nd harvest time (). The N, P, K, Ca values of tea leaves varied from 4.61 to 3.73%; 0.21 to 0.35%; 1.43 to 1.97%; and 0.26 to 0.31%, respectively (). Mg, S and Mn values of tea leaves at different harvest times are determined as 0.23 to 0.28%; 0.20 to 0.25% and 0.04 to 0.12%, respectively ().
Table 2. Mineral content of tea leaves.
The mineral composition of plants depended, not only on the species or varieties, but also on the growing conditions such as soil and geographical condition. We used only one cultivar in this study. The research area was same as well. Therefore main effect on mineral content should be harvest times as mentioned above. In this study, while the existence of seven elements was determined in all harvest times, N was predominant, followed by K, Ca, and P, respectively (). It is previously reported that N content of tea leaves were between 3.0-4.0% (CitationOwuor & Wanyoka, 1983) which in agreement with our results. CitationKacar et al. (1979) reported that N content of tea leaves was highest at 1st harvest and decreased to last harvest which supports our findings. CitationOzgumus et al. (1982) found that P content of tea leaves were between 0.31-0.40%. Our P results are lower than this literature. This could be explained of low P content of soils. It is previously reported that approximately 30% of tea soils in Black Sea Region in Turkey had P deficiency (CitationSarimehmet, 1989). In addition, our K results are in accordance with CitationKacar et al. (1979).
As a conclusion of this study, it can be said that tea leaves are a valuable product, based on its rich and beneficial nutrient composition.
References
- Anon. (2000): Sherlock Microbial Identification System. Version 4 MIS operating manual, Newark, DE, USA. pp. 66–89.
- Anon. (2005): Food and Agricultural Organization. www.fao.org.
- P D Duh, Y Y Tu, and G C Yen. (1999). Antioxidant activity of aqueous extract of harn jyur (Chyrsanthemum morifolium Ramat). Lebensmittel-Wissenschaft und Technologie 32:269–277.
- P L Fernandez, F Pablos, M J Martin, and A G Gonzalez. (2002). Multi-element analysis of tea beverages by inductively coupled plasma atomic emission spectrometry. Food Chem 76:483–489.
- L Ferrara, D Montesano, and A Senatore. (2001). The distribution of minerals and flavonoides in the tea plant. II Farmaco 56:397–401.
- Y Hara, S J Luo, R L Wickremasinghe, and T Yamanishi. (1995). Special issue on tea. Food Rev. Int 11:371–545.
- M E Harbowy, and D A Balentine. (1997). Tea chemistry. Crit. Rev. Plant Sci 16:415–480.
- N Ito, S Fukushima, A Hassegawa, M Shibata, and T Ogiso. (1983). Carcinogenicity of butylated hydroxyanisole in F344 rats. J Nat Cancer Inst 41:215–217.
- G S James (1995): Analytical Chemistry of Foods. London: Blackie Academic and Professional, pp. 117–120.
- H R Juliani, and J E Simon (2002): Antioxidant activity of Basil. Trends in new crops and new uses ( Eds, J. Janick, and A. Whipkey). ASHS Press 575–579.
- B Kacar, E Przemeck, A Ozgumus, C Turan, A V Katkat, and I Kayikcioglu (1979): A study on the microelement necessity of tea plants and soils. Tubitak, TOAG-321, Ankara, 1–67.
- C Kaur, and H C Kapoor. (2002). Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol 37:153–161.
- A Y Leung, and S Foster (1996): Encyclopedia of common natural ingredients used in food, drugs, and cosmetics. (New York: John Wiley & Sons, Inc.). 489–491 (2nd ed.).
- K Mendilcioglu (2000): Tea growth techniques. Ege University Agricultural Faculty, No: 12, pp. 43.
- H Mukhtar, and N Ahmad. (2000). Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 71 (6 Suppl):1698S–1702S. (discussion 1703S–1694S)
- M Naczk, and F Shahidi. (1989). The effect of methanol-ammonia-water treatment on the content of phenolic acids of canola. Food Chem 31:159–164.
- M Obanda, P O Owuor, and C K Njuguna. (1992). The impact of clonal variation of total polyphenols content and polyphenol oxidase activity of fresh tea shoots on plain black tea quality parameters. Tea 13:129–133.
- M Obanda, P O Owuor, and S J Taylor. (1997). Flavanol composition and caffeine content of green leaf as quality potential indicators of Kenyan black teas. J. Sci. Food Agric 74:209–215.
- P O Owuor, and J K Wanyoka. (1983). Fertilizer use advisory service a reminder to farmers. Tea 3 (2):3–7. 1983
- A Ozgumus, C Turan, and B Kacar. (1982). The phosphorus situation of tea soils in Turkey. Doga 6:201–203.
- R J Pawlosky, G Ward, and N Salem. (1996). Essential fatty acid uptake and metabolism in the developing rodent brain. Lipids 31 (suppl):S103–S107.
- A Robertson (1992): The chemistry and biochemistry of black tea production—the nonvolatiles. In: K. C. Willson (ed.) and M. N. Clifford, Tea: Cultivation to Consumption. Kluwer Acad. Publ., Dordrecht, The Netherlands. pp. 555–601.
- M Sarimehmet (1989): Fertilization Problems in Tea and Solutions. Caykur Publication No. 13, Ankara University Press, 49 p. (in Turkish).
- M Serafini, A Ghiselli, and A Ferro-Luzzi. (1996). In vitro antioxidant effect of green and black tea in man. Eur J Clin Nutrition 50:28–32.
- F Shahidi, and P KJ Wanasundara. (1992). Phenolic antioxidants. Critical Rev Food Sci Nutrition 32:67–103.
- A P Simopoulos, and N Salem. (1996). Fatty acids and lipids from cell biology to human disease. Lipids 31 (suppl):S1–S1.
- I Tavazzi, and E Offord. (2001). Comparison of the antioxidant activity of commonly consumed polyphenolic beverages (coffee, cocoa, and tea) prepared per cup serving. J Agr Food Chem 49:3438–3442.
- N Turkmen, F Sari, and Y S Velioglu. (2006). Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chem 99:835–841.
- C S Yang, P Maliakal, and X Meng. (2002). Inhibition of carcinogenesis by tea. Ann Rev Pharmacol Toxicol 42:25–54.
- N T Zaveri. (2006). Green tea and its polyphenolic catechins: Medicinal uses in cancer and noncancer applications. Life Sciences 78:2073–2080.
- M Xie, A Xie Von Bohlen, R Klockenkamper, X Jian, and K Gunther. (1998). Multielement analysis of chinese tea (Camellia sinensis L.) by total reflection X ray fluorescence. Z. Lebensm Unters Forsch A. 207:31–38.