Abstract
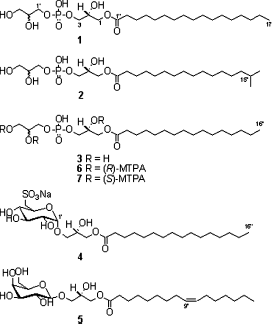
A chemical investigation of the MeOH extract of a two-sponge association (Jaspis sp. and Poecillastra sp.), collected from Jeju Island, Korea, led to the isolation of two new lysophosphatidylglycerols (1 and 2) along with known lysophosphatidylglycerol (3) and glycolipids (4 and 5). Their structures were elucidated on the basis of NMR and MS spectroscopic data.
Introduction
Sponges of the genus Jaspis are reported to contain many biologically active molecules, which include macrolides [jaspisamides (CitationKobayashi et al., 1993)], cyclic peptides [jaspamides (CitationGala et al., 2007) or jasplakinolide (CitationCrews et al., 1986)], amino acid derivatives [bengamides (CitationThale et al., 2001) and bengazoles (CitationGroweiss et al., 1999)], and triterpenes (CitationTang et al., 2006). Similarly, macrolide lactams [poecillastrins (CitationTakada et al., 2007)] and sesquiterpene derivatives (CitationKillday et al., 1993) have been isolated from sponges of the genus Poecillastra. Two-sponge association of Jaspis sp. and Poecillastra sp. was known to contain bisimidazoles [wondonins (CitationShin et al., 2001)].
As a part of our continuing research aimed at the discovery of biologically active secondary metabolites from marine organisms (CitationMansoor et al., 2007a,Citationb; CitationShinde et al., 2007), we had isolated pectenotoxin II and psammaplins from the 90% MeOH extract of a two-sponge association of Jaspis sp. and Poecillastra sp. by bioactivity-guided fractionation (CitationJung et al., 1995; CitationPark et al., 2003). In a continuing study, two new (1 and 2) and one known (3) lysophosphatidylglycerols and two known glycolipids (4 and 5) were isolated from the MeOH extract. In this paper, we describe the isolation and structural elucidation of 1– 5 ().
Materials and Methods
General experimental methods
Optical rotations were measured with a Jasco (Japan) P-1020 polarimeter using a 1-dm path length cell. The1H and 2D NMR spectra were recorded at 500 MHz using a Varian INOVA (USA) 500 spectrometer. The13C NMR spectra were recorded at 125 MHz and 75 MHz using Varian INOVA 500 and Varian UNITY 300 spectrometers. Fast Atom Bombardment Mass Spectrometry (FABMS) data were obtained on a JEOL (USA) JMS SX-102A spectrometer. High Resolution Fast Atom Bombardment Mass Spectrometry (HRFABMS) data were obtained on a JEOL JMS SX-101A spectrometer. Chemical shifts were reported with reference to the respective solvent peaks and residual solvent peaks (δH 3.30 and δC 49.0 for CD3OD). HPLC was performed on a Gilson (USA) 370 pump with a Shodex C18M 10E (preparative, 250 × 10 mm, i.d., 5 μm, and 100 Å) column using Shodex RI-71 and RI-101 detectors.
Animal material
The sponges, collected in November 2002 off the Coast of Jeju Island, South Korea, using SCUBA, were frozen immediately after collection and stored at −20°C until extraction. This specimen was identified as an association of sponges Jaspis sp. (Jaspidae) and Poecillastra sp. (Pachastrellidae) by Prof. Chung Ja Sim of Hannam University. A voucher specimen was deposited in the Natural History Museum, Hannam University, Daejon.
Extraction and isolation
The freeze-dried animal material was cut into small pieces and extensively extracted with MeOH at room temperature. The MeOH extract showed toxicity against brine shrimp larvae (LD50 43 μ g/mL). The MeOH extract was partitioned between CH2Cl2 and H2O. The CH2Cl2 layer was further partitioned between 90% MeOH and n-hexane. The aqueous MeOH fraction was selected for further separation on the basis of its toxicity (LD50 16 μ g/mL) in brine shrimp lethality assay (CitationMeyer et al., 1982) and subjected to a reversed-phase flash column chromatography (YMC Gel ODS-A, 60 Å 500/400 mesh), eluting with a gradient solvent system of 50% to 100% MeOH to yield 19 fractions (DCM1–DCM19). Fractions DCM11 to DCM16 were subjected to repeated reversed-phase chromatographic separation to afford 10 compounds. Compound 1 was obtained from fraction DCM13 and compound 2 was isolated from fraction DCM14. Compounds 3 and 4 were obtained from fraction DCM11, and fraction DCM16 yielded compound 5.
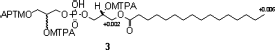
Methoxy-trifluoromethyl-phenyl-acetate (MTPA) ester of compound 3: Each 0.3 mg of compound 3 (0.61 μ mol) was dissolved in CDCl3 (300 μL), followed by treatment with (R)-(−)- and (S)-(+)-α -methoxy-α -(trifluoromethyl)phenylacetyl chloride (0.46 μL) in pyridine-d5 (10 μL) to yield (S)-MTPA ester (6) and (R)-MTPA ester (7), respectively. After being kept at room temperature for 24 h, the reaction mixtures were evaporated and NMR spectra measured.
1-O-Heptadecanoyl-sn-glycero-3-phosphoglycerate (1): Light yellow oil; [α]23D −5.4° (c 0.10, MeOH); 1H NMR (CD3OD, 500 MHz) δ 4.16 (1H, dd, J = 12.0, 5.0 Hz, H-1a), 4.09 (1H, dd, J = 12.0, 5.0 Hz, H-1b), 3.96 (1H, m, H-2), 3.88 (4H, m, H-3, H-1′), 3.75 (1H, m, H-2′), 3.59 (1H, dd, J = 12.0, 5.5 Hz, H-3′a), 3.54 (1H, dd, J = 12.0, 5.5 Hz, H-3′b), 2.34 (2H, t, J = 7.5 Hz, H-2″), 1.60 (2H, m, H-3″), 1.25–1.31 (26H, br s, H-4″–H-16″), 0.89 (3H, t, J = 7.0 Hz, H-17″); 13C NMR (CD3OD, based on HSQC and HMBC experiments, 500 MHz) δ 174.0 (C-1″), 71.3 (C-2′), 68.7 (C-2), 66.3 (C-3), 66.3 (C-1′), 65.0 (C-1), 62.6 (C-3′), 33.6 (C-2″), 29.4–32.8 (C-4″–C-16″), 24.6 (C-3″), 12.8 (C-17″); LRFABMS m/z 497 [M – H]−; HRFABMS m/z 497.2865 [M – H]− (calcd for C23H46O9P, 497.2879).
1-O-(15-M e t h y l h e x a decanoyl)-sn-glycero-3-phospho-glycerate (2): Light yellow oil; [α]22D –0.88° (c 0.09, MeOH); 1H NMR (CD3OD, 500 MHz) δ 4.16 (1H, dd, J = 12.0, 5.0 Hz, H-1a), 4.09 (1H, dd, J = 12.0, 5.0 Hz, H-1b), 3.96 (1H, m, H-2), 3.88 (4H, m, H-3, H-1′), 3.75 (1H, m, H-2′), 3.59 (1H, dd, J = 12.0, 5.5 Hz, H-3′a), 3.54 (1H, dd, J = 12.0, 5.5 Hz, H-3′b), 2.34 (2H, t, J = 7.5 Hz, H-2″), 1.60 (2H, m, H-3″), 1.52 (1H, m, H-15″), 1.25–1.31 (20H, br s, H-4″–H-13″), 1.16 (2H, m, H-14″), 0.86 (6H, d, J = 6.5 Hz, H-16″, H-17″); 13C NMR (CD3OD, based on Heteronuclear Single Quantum Coherence (HSQC) and Heteronuclear Multiple Bond Correction (HMBC) experiments, 500 MHz) δ 174.0 (C-1″), 71.3 (C-2′), 68.7 (C-2), 66.3 (C-3), 66.3 (C-1′), 65.0 (C-1), 62.6 (C-3′), 38.0 (C-14″), 33.6 (C-2″), 29.4–32.8 (C-4″–C-13″), 28.8 (C-15″), 24.6 (C-3″), 19.9 (C-16″, 17″); Low Resolution Fast Atom Bombardment Mass Spectrometry (LRFABMS) (− ve mode) m/z 497 [M – H]−, (+ ve mode) m/z 521 [M + Na]+, m/z 543 [M + 2Na − H]+;HRFABMS m/z 543.2656 [M + 2Na − H]+(calcd. for C23H46O9PNa2, 543.2675).
Results and Discussion
The MeOH crude extract of a two-sponge association of Jaspis sp. and Poecillastra sp. was subjected to activity-guided fractionation using solvent partition and reversed-phase flash column chromatography followed by repeated reversed-phase HPLC to yield compounds 1– 5. The structures of these metabolites were deduced using NMR (1H, 13C, COSY, HSQC, and HMBC) and MS analysis, optical rotation data, and by direct comparison with those reported.
Compound 1 was isolated as light yellow oil. Its molecular formula was established as C23H47O9P on the basis of HRFABMS and NMR data. The exact mass of the [M – H]− ion (m/z 497.2865) matched well with the expected molecular formula C23H46O9P (Δ − 1.4 mmu). The 1H NMR spectrum of 1 displayed signals typical for glycerol moieties, which included multiplets at δ 3.96 (H-2), 3.75 (H-2′), and 3.88 (H2-3, H2-1′), and doublets of doublet at δ 4.16 (H-1a), 4.09 (H-1b), 3.59 (H-3′a), and 3.54 (H-3′b). The presence of two glycerol moieties was also confirmed from the analysis of COSY spectrum. A large peak at δ 1.28–1.40 indicated the presence of linear aliphatic hydrocarbon chain that was coupled with methyl signal at δ 0.89 (t, J = 7.0 Hz, H3-17″) in the COSY spectrum. The 13C NMR data of six oxygenated carbons at δ71.3 (C-2′), 68.7 (C-2), 66.3 (C-3), 66.3 (C-1′), 65.0 (C-1), 62.6 (C-3′) in conjunction with one ester carbonyl group at δ 174.0 (C-1″), aliphatic alkyl chain at δ 24.6–33.6 (C-2″–C-16″), and one methyl carbon at δ 12.8 (C-17″) supported the above assignments. Furthermore, HMBC correlations from H-1a (δ 4.16) and H-1b (δ 4.09) to the carbonyl carbon at C-1″ (δ 174.0) confirmed the ester linkage in the molecule. The presence of phosphate group was secured from the careful comparison of obtained NMR data with those reported (CitationTamehiro et al., 2002). The absolute configuration at C-2 was presumed to be R (vide infra). Thus, the chemical structure of compound 1 was established as 1-O-heptadecanoyl-sn-glycero-3-phosphoglycerate. To our knowledge, this is the first report on isolation of lysophosphatidylglycerol containing heptadecanoic acid moiety.
Compound 2 was isolated as light yellow oil. The HRFABMS of 2 supported the molecular formula C23H47O9P, the same as 1. The exact mass of the [M + 2Na − H]+ ion (m/z 543.2656) matched well with the expected molecular formula C23H46O9PNa2 (Δ − 1.9 mmu). The negative and positive FABMS spectra of 2 showed the [M – H]−, [M + Na]+ and [M + 2Na − H]+ ions at m/z 497, 521, and 543, respectively. 1H and 13C NMR data of 2 were almost identical to those of 1, with only difference in signals of the fatty acid residue. The 1H NMR spectrum of 2 exhibited signals for an isopropyl group (CitationMansoor et al., 2005), indicated by a two-methyl doublet at δ 0.86 (J = 6.5 Hz, H3-16″, H3-17″), which in turn showed coupling with a methine signal at δ 1.52 (H-15″), which was coupled with methylene protons at δ 1.16 (H2-14″) in the COSY spectrum. The absolute configuration at C-2 was presumed to be R (vide infra). Hence, compound 2 was deduced to be 1-O-(15-methylhexadecanoyl)-sn-glycero- 3-phospho-glycerate. Compound 2 represents the first example of lysophosphatidylglycerol containing terminal isopropyl group.
Known metabolites, lysophosphatidylglycerol (3), α-quinovosylglycerol (4), and β-galactosylglycerol (5), were also isolated in the current study. Their structures were identified by comparison of NMR and MS data with those reported (CitationRho et al., 1996; CitationSakamoto et al., 2000; CitationYu et al., 2006).
The absolute stereochemistry at C-2 in compound 3 was determined as R by Mosher′s method (CitationDale & Mosher, 1973). The (S)- and (R)-MTPA esters (6 and 7) were prepared and Δ δ (δS−δR) values for all assignable protons were observed (). However, the absolute configuration at C-2′ could not be defined due to hidden signals of H2-3′ with MTPA signals. As the chemical shift values of 1 and 2 are similar with that of compound 3, and from the same sign (−ve) of optical rotation, the same stereochemistry (2R) can be assumed for compounds 1 and 2.
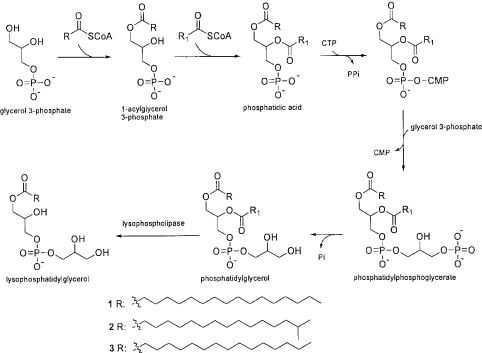
Lysophosphatidylglycerols may be biosynthesized from phosphatidylglycerol through acyl ester hydrolysis as last step () (CitationDe et al., 1993; CitationTamehiro et al., 2002). Glycerol 3-phosphate upon esterification with the first fatty acyl-CoA gives 1-acylglycerol 3-phosphate, which undergoes esterification with the second fatty acyl-CoA to form phosphatidic acid (1,2-diacylglycerol 3-phosphate). This phosphatidic acid will be converted into phosphatidylphosphoglycerate on addition of a glycerol 3-phosphate molecule, which forms phosphatidylglycerol upon removal of a phosphate molecule. Enzyme lysophospholipase carries out hydrolysis of phosphatidylglycerol to yield lysophosphatidylglycerol (CitationDe et al., 1993; CitationTamehiro et al., 2002).
Bacilysocin, a novel lysophosphatidylglycerol, isolated from Gram-positive bacteria (Bacillus subtilis), is reported to possess antimicrobial activity against bacteria and certain fungi (CitationTamehiro et al., 2002). One recent study showed that lysophosphatidylglycerols inhibit β -glucuronidase and can be used for prevention of colon and bladder cancers and also as drugs (oral or topical), health foods, and cosmetics for inhibiting unpleasant body odor (Shiojiri, Citation2007). Small amounts of the isolated metabolites (1– 3) prevented us from carrying out any biological evaluation.
Phosphatidylglycerols are usually major components of phospholipids in microorganisms. Lysophosphatidylglycerols are common components of bacterial membrane and, as we know, there is a close relationship between sponges and bacteria; the isolation of these types of compounds from the methanol extract of a two-sponge association of Jaspis sp. and Poecillastra sp. is not surprising and might be the result of presence of bacteria in sponge bodies. Therefore, it would be interesting to study metabolites of the bacteria associated with these sponges to determine the real producer of interesting metabolites such as macrolides, peptides, and psammaplins that were previously reported from these sponges.
Acknowledgements
This study was financially supported by a grant from Marine Bio 21, Ministry of Maritime Affairs and Fisheries, Korea. Our thanks are due to Prof. Chung Ja Sim of Hannam University for the identification of the sponge material.
References
- P Crews, L V Manes, and M Boehler. (1986). Jasplakinolide, a cyclodepsipeptide from the marine sponge, Jaspis sp. Tetrahedron Lett 27:2797–2800.
- J A Dale, and H S Mosher. (1973). Nuclear magnetic resonance enantiomer regents. Configurational correlations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmandelate, and α-methoxy-α-trifluoromethyl-phenylacetate (MTPA) esters. J Am Chem Soc 95:512–519.
- M D De, R Grau, and J EJ Cronan. (1993): Cell Envelope In: AL Soneshein, J A Hoch, and R Losick. eds., Bacillus subtilis and Other Gram-Positive Bacteria; Washington, DC, American Society for Microbiology; pp. 411–423.
- F Gala, M V D'Auria, S D Marino, F Zollo, C D Smith, J E Copper, and A Zampella. (2007). New jaspamide derivatives with antimicrofilament activity from the sponge Jaspis splendans. Tetrahedron 63:5212–5219.
- A Groweiss, J J Newcomer, B R O'Keefe, A Blackman, and M R Boyd. (1999). Cytotoxic metabolites from an Australian collection of the sponge Jaspis species. J Nat Prod 62:1691–1693.
- J H Jung, C J Sim, and C O Lee. (1995). Cytotoxic compounds from a two sponge association. J Nat Prod 58:1722–1726.
- K B Killday, R Longely, P J McCarthy, S A Pomponi, A E Wright, R F Neale, and M A Sills. (1993). Sesquiterpene-derived metabolites from the deep water marine sponge Poecillastra sollasi. J Nat Prod 56:500–507.
- J Kobayashi, O Murata, H Shigemori, and T Sasaki. (1993). Jaspisamides A–C, new cytotoxic macrolides from the Okinawan sponge Jaspis sp. J Nat Prod 56:787–791.
- T A Mansoor, B K Bae, J Hong, C O Lee, K S Im, and J H Jung. (2005). New fatty acid derivatives from Homaxinella sp., a marine sponge. Lipids 40:981–985.
- T A Mansoor, P B Shinde, X Luo, J Hong, C O Lee, C J Sim, B W Son, and J H Jung. (2007a). Renierosides, cerebrosides from a marine sponge Haliclona (Reniera) sp. J Nat Prod 70:1481–1486.
- T A Mansoor, T Park, X Luo, J Hong, C O Lee, and J H Jung. (2007b). A new spingosine from a marine sponge Haliclona (Reniera) sp. Nat Prod Sci 13:247–250.
- B N Meyer, N R Ferrigni, J E Putnam, L B Jacobsen, D E Nichols, and J L McLaughlin. (1982). Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med 45:31–34.
- Y Park, Y Liu, J Hong, C O Lee, H Cho, D K Kim, K S Im, and J H Jung. (2003). New bromotyrosine derivatives from an association of two sponges, Jaspis wondoensis Poecillastra wondoensis. J Nat Prod 66:1495–1498.
- M C Rho, K Matsunaga, K Yasuda, and Y Ohizumi. (1996). A novel inhibitor from the Cynophyceae Oscillatoria rosea (NIES-208). Planta Med 62:473–474.
- B Sakamoto, Y Hokama, F D Horgen, P J Scheuer, Y Kan, and H Nagai. (2000). Isolation of a sulfoquinovosyl monoglycerol from Bryopsis sp. (Chlorophyta): Identification of a factor causing a possible species-specific ecdysis response in Gambirdiscus toxicus (Dinophyceae). J Phycol 36:324–931.
- P B Shinde, T A Mansoor, X Luo, J Hong, C O Lee, and J H Jung. (2007). Cytotoxic polyketides from the marine sponge Discodermia calyx. Bull Korean Chem Soc 28:990–994.
- J Shin, J R Rho, Y Seo, H S Lee, K W Cho, H J Kwon, and C J Sim. (2001). Wondonins A and B, new bis(diydroxystyryl)imidazoles from a two-sponge association. Tetrahedron Lett 42:1965–1968.
- M Shiojiri 2007: Lysophospholipids from natural products as β -glucuronidase inhibitors. Jpn Kokai Tokkyo Koho JP 20071768689 pp.
- K Takada, B W Choi, M A Rashid, W R Gamble, I IJH Cardellina, Q N Van, J R Lloyd, J B McMahon, and K R Gustafson. (2007). Structural assignment of poecillastrins B and C, macrolide lactams from the deep-water caribbean sponge Poecillastra species. J Nat Prod 70:428–431.
- N Tamehiro, H Y Okamoto, S Okamoto, M Ubukata, M Hamada, H Naganawa, and K Ochi. (2002). Bacilysocin, a novel phospholipid antibiotic produced by Bacillus subtilis 168. Antimicr Agents Chemother 46:315–320.
- S Tang, Y Pei, H Fu, Z Deng, J Li, P Proksh, and W Lin. (2006). Jaspolides A–F, six new isomalabaricane–type terpenoids from the sponge Jaspis sp. Chem Pharm Bull 54:4–8.
- Z Thale, F R Kinder, K W Bair, J Bontempo, A M Czuchta, R W Versace, P E Phillips, M L Sanders, S Wattanasin, and P Crews. (2001). Bengamides revisited: New structures and antitumor studies. J Org Chem 66:1733–1741.
- S Yu, N Fang, Q Li, J Zhang, H Luo, M Ronis, and T M Badger. (2006). In vitro actions on human cancer cells and the liquid chromatography-mass spectrometry/mass spectrometry fingerprint of phytochemicals in rice protein isolate. J Agri Food Chem 54:4482–4492.