Abstract
Piper carpunya Ruiz & Pav. (Piper lenticellosum C. DC.), a member of the Piperaceae family, is a remedy with anti-inflammatory and antiulcer properties that is used in folk medicine in Ecuador. The present work determined the gastroprotective effects of this plant in a model of diclofenac-induced ulcers in rats and studied the mechanism involved. The aqueous suspension of the ethanol extract of the leaves of P. carpunya was administered twice orally to three groups of Wistar rats at doses of 62.5, 125, and 250 mg/kg, with a 24-h interval between doses. Diclofenac (100 mg/kg) was given 1 h after the last administration of the extract. Pretreatment with P. carpunya decreased the ulcerated area, prevented neutrophil infiltration induced by NSAID administration in vivo, and inhibited the release of the proteolytic enzyme myeloperoxidase by neutrophils stimulated with the calcium ionophore A23187. Superoxide dismutase activity demonstrated a marked increase at the two lowest doses of the extract versus diclofenac group and glutathione peroxidase activity was reverted near to sham levels. On the other hand, P. carpunya treatment did not prevent the increased levels of lipoperoxides in gastric mucosa caused by diclofenac. Furthermore, P. carpunya did not affect PGE2 levels, which were depleted by diclofenac treatment. These results suggest that the gastroprotective effect of P. carpunya in this experimental model appears through anti-inflammatory and antiradical mechanisms.
Introduction
Gastrointestinal diseases are among the most common pathologies that require pharmacological treatment. Among the factors that can cause gastric pathologies are non-steroidal anti-inflammatory drugs (NSAID). These drugs are used worldwide for treatment of pain, rheumatic (CitationBjordal et al., 2004) and cardiovascular diseases (CitationBredie et al., 2003), and they also have been shown to protect against colon cancer (CitationWang & Zhang, 2005) and Alzheimer disease (CitationAizen et al., 2005). Unfortunately, these drugs cause mucosal injury in the gastrointestinal tract and occasionally give rise to severe complications, such as bleeding and perforation (CitationSakamoto, 2003). In an attempt to reduce the incidence of NSAID-induced gastropathy, the following approaches have been proposed: avoidance of NSAIDs or minimising their dosage, selecting NSAIDs known to cause less damage and co-prescription of various agents. In developing countries, the population continues to use traditional medicine for NSAID-induced gastrointestinal problems. However, during the past decade, a huge amount of research work has focused on the scientific evaluation of traditional drugs of plant origin (CitationLans & Brown, 1998; CitationGrover & Yadav, 2004). Currently, many countries with important biodiversity resources are developing and using non-toxic preparations from traditional medicinal plants for controlling various diseases, providing relief of symptoms comparable to that obtained from allopathic medicine. Thus, novel non-toxic, antisecretory, and antiulcer preparations from medicinal plants are demanded as alternative treatment for the efficient management of gastric hypersecretion and gastroduodenal ulcers (CitationAlarcon de la Lastra et al., 1994; Martín et al., 1996; CitationBandyopadhyay et al., 2004; CitationTapia et al., 2004).
Piper carpunya Ruiz & Pav. (P. lenticellosum C. DC.) (Piperaceae), known by the vulgar name of “guaviduca”, is widely used in folk medicine in Ecuador as an anti-inflammatory and antiulcer remedy. There are not many reports about the mechanisms involved in the gastroprotective effect of this plant, but the variety from Ecuador has been investigated in order to validate the anti-inflammatory activity in vivo, and the results obtained support its use in traditional medicine in this country (Citationde las Heras et al., 1998). These facts justify our interest in further studies on this plant.
For this reason, this study elucidated the gastroprotective effect of the ethanol extract from the leaves of P. carpunya in a model of diclofenac-induced gastric ulcers in rats and attempted to elicit the underlying mechanisms. We studied the role of this extract on oxidative stress in gastric mucosa by measuring changes in glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) activity, as well as the inhibition of lipid peroxidation. Prostaglandin E2 (PGE2) levels in gastric mucosa were also evaluated. Furthermore, we studied myeloperoxidase (MPO) activity in vivo as a marker of neutrophil infiltration and in vitro as an assay for enzyme inhibition. Moreover, we investigated scavenging of hydrogen peroxide in order to assess the mechanism involved.
Materials and Methods
Plant material
For the present experiments, the leaves of P. carpunya were collected in May 2004 in the province of Pichincha in the central region of Ecuador, and identified by Dr. Cerón. Voucher specimens of this species are kept under reference 186 in the Department of Botany, Biology Faculty of the Central University of Ecuador.
Preparation of the extract
Fresh leaves were dried at room temperature for 10–14 days in a room without direct sunlight. Then the leaves were powdered and stored at −10°C until used. The powdered leaves (500 g) were extracted at room temperature with ethanol 96% during 24–48 h by maceration. The suspension was filtered and concentrated to half volume under reduced temperature and pressure using a rotary evaporator. The residue was percolated with ethanol used for the maceration and new ethanol was also added (exhaustive extraction). Afterwards, this extract was concentrated again at 45°C to half volume. The last residue of leaves was mixed with this concentrate and the resultant extract was dried in a rotary evaporator. The total volume of ethanol was 31 mL and the yield of the concentrate was 4.28%. For in vivo studies, the plant extract was suspended in water; for the in vitro studies, it was suspended in aqueous ethanol (70%).
Animals
Male and female Wistar rats (180–200 g), kept in standard laboratory conditions, were fasted overnight in single wire net floor cages with free access to tap water, and randomly assigned to groups of 10–12 animals. All experiments followed a protocol approved by the local animal ethics committee and the local government and were in accordance with the recommendations of the European Union.
In vivo experimental protocols
Dose selection and administration route
Three different doses of the ethanol extract, 62.5, 125, and 250 mg/kg body weight, were used in the present study. The aqueous suspension of the above mentioned extract of P. carpunya (in a volume of 1 mL/100 g body weight) was prepared freshly each time and administered twice each to the different animal groups at 24-h interval by oral gavage. The doses were selected according to previous experiments of our group (CitationBerenguer et al., 2006).
Ulcer induction
Diclofenac (Sigma Chemical Co. Madrid, Spain) was used as ulcerogenic agent at a dose of 100 mg/kg. One h after the last administration of the plant extract animals received diclofenac 100 mg/kg by the same oral route. Rats were sacrificed by cervical dislocation 6 h after NSAID administration. Vehicle-treated (sham) and diclofenac-treated rats were included as controls in all experiments. Experimental times were selected according to former experiments of our group that reflect the damage induced by the assayed NSAID peaks 6 h after oral administration (CitationSánchez et al., 2002).
Ulcer assessment
The stomachs were harvested and opened along the greater curvature, and the mucosa exposed for macroscopic evaluation. The ulcerated area was assessed using planimetry by a person unaware of the type of treatment received by the animals, and the ulcer index (UI) (mm2) was calculated as the arithmetic mean for each treatment. Following the analysis, the mucosa layer was blotted dry, and scraped off the underlying muscularis externa and serosa. A homogeneous mixture of mucosa, damaged and macroscopically healthy tissue, was snap-frozen in liquid nitrogen, and stored at −70°C before biochemical studies.
Histological evaluation
Ulcerated portions from 2–3 stomachs of each experimental group were cut out with a scalpel and were fixed for 4 h in 4% buffered paraformaldehyde, then dehydrated gradually in ethanol and embedded in paraffin using xylene as intermediate solvent. Serial sections (6 μ m) were obtained by cutting the block in a plane perpendicular to the mucosal surface with a microtome. Coded gastric sections were stained with hematoxylin and eosin before light microscope evaluation.
Myeloperoxidase activity
MPO activity was assessed as a marker of neutrophil infiltration according to the method of CitationGrisham et al. (1990). The tissue was homogenized in phosphate-buffered saline (PBS), pH = 7.4 and centrifuged, and the pellet was again homogenized in PBS, pH = 6.0, containing hexadecyl-trimethylammonium bromide (HETAB) and ethylendiamine tetra-acetic acid. This homogenate was subjected to one cycle of freezing/thawing and a brief period of sonication. A sample of homogenate (0.5 μ L) was added to a 0.5 mL reaction volume containing PBS, pH 5.4, HETAB and 3,3′,5,5′-tetramethylbenzidine. The mixture was incubated at 37°C. The reaction was started by the addition of H2O2, and was terminated by the sequential addition of catalase and sodium acetate, pH = 3.0. The changes in absorbance at 655 nm were measured with a microplate reader (Labsystem Multiskan Ex). One unit of MPO activity was defined as the amount of enzyme present that produced a change in absorbance of 1.0 U/min at 37°C, and the results were expressed as U/mg tissue.
Lipid peroxidation
The levels of thiobarbituric acid reactants (TBARS) in the gastric mucosa as index of lipoperoxide production, were measured according to the modified method from CitationOhkawa et al. (1979). The mucosa was scraped with glass slides, weighed, and homogenized in 10 mL KCl (10%). The homogenate was supplemented with 8.1% sodium lauryl sulfate, 20% acetic acid, and 0.8% TBA, and boiled at 100° C for 1 h. After cooling, the reactants were supplemented with 2.5 ml n-butanol, shaken vigorously for 1 min, and centrifuged for 10 min at 2600 g. Absorbance was measured in a spectrophotometer (Perkin-Elmer Lambda 3) at 532 nm, and the results were expressed as nanomoles of malonyldialdehyde (MDA) per g of tissue.
Superoxide dismutase activity
The enzymatic activity of SOD is based on the inhibition of the reduction of cytochrome c according to the method of CitationMcCord and Fridovich (1969). Samples of gastric mucosa were homogenized (1:150) in a mixture of 50 mM PBS and 100 μ M EDTA (pH 7.8). The homogenate was supplemented with 0.1% Triton. The assay method used 10 μ M ferricytochrome c, 50 μ M xanthine, as source of O2−, and sufficient milk xanthine oxidase (5 nM) to give a rate of increase in absorbance of 0.025/min at pH 7.8 and 25°C. The reaction kinetic was measured in a spectrophotometer at 550 nM at a rate of 0–70 s. Results were expressed as U SOD/mg tissue. One unit of SOD is defined as the amount of enzyme that causes 50% inhibition of cytochrome c reduction.
Glutathione peroxidase activity
Glutathione peroxidase (GSH-Px) activity was quantified by the method of CitationYoshikawa et al. (1993). The reaction mixture consisted of 50 mM PBS, pH 7, 1 mM EDTA, 1 mM NaN3, 0.2 mM NADPH, 1 unit/mL oxidized GSSG-Rd in PBS buffer, pH 7.8, 1 mM GSH and 0.25 mM H2O2. Samples were added to 0.8 mL of the above mixture and incubated for 5 min at 25°C before initiating the reaction with the addition of peroxide solution. A sample of supernatant fluid with 10% homogenate solution and 1.15% KCl was prepared by centrifugation at 4000 g for 10 min at 4°C. The absorbance at 340 nm was recorded for 5 min. The activity was the slope of the lines as micromoles of NADPH oxidized per min. The blank datum (the enzyme was replaced with distilled water) was subtracted from each value. Results were expressed as nanomoles per min per mg of tissue.
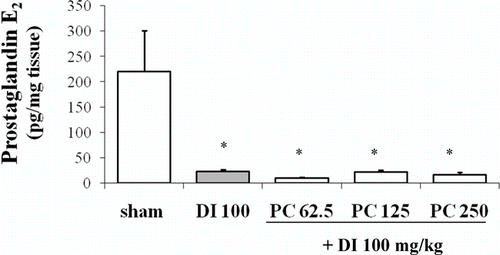
Prostaglandin E2 levels
Gastric mucosa from the different animal groups was excised and rapidly rinsed with ice-cold saline. The tissue was weighed and homogenized in 6 mL TEAP buffer (pH 3.24) which contained a cyclooxygenase inhibitor, lysine acetyl salicitate (Inyesprin®, Grünenthal). The homogenate was centrifuged (3000 rpm, 10 min, 4°C) and the supernatant was removed and passed through a reverse-phase octadecylsilica C18 Sep Pak cartridge which was washed with 10 mL distilled water, 10 mL 15% ethanol, 10 mL hexane and 10 mL ethyl acetate, and the eluate collected. Each fraction with ethyl acetate was evaporated, and the dry residue redisolved in ethanol. PGE2 was determined by a competitive enzyme immunoassay kit (Cayman®, Cat. No. 514010). Results were expressed as pg PGE2/mg tissue.
In vitro experimental protocols
Leukocyte isolation
A suspension of leukocytes containing approximately 85% polymorphonuclear leukocytes (PMNs) and 15% mononuclear cells was obtained from male Wistar rats (200–300 g) by an intraperitoneal injection of 10 mL of a solution of 6% oyster glycogen in saline followed 16–20 h later by 60 mL ice-cold modified Hank's balanced salt solution (HBSS) free of Ca2 + and Mg2 + (CitationMoroney et al., 1988).The mixed peritoneal leukocytes were resuspended in complete HBSS at 2.5 × 106 cells/mL containing 1.26 mM Ca2 +and 0.9 mM Mg2 +. Cell viability, based on Trypan blue exclusion, was greater than 95%.
Myeloperoxidase released from stimulated rat peritoneal leukocytes
Triplicate aliquots of 0.5 mL leukocytes were preincubated at 37°C for 10 min with 5 μ L of an ethanol plant extract (the assayed doses were 50 and 25 μ g/μ L) or an equivalent volume of the vehicle (ethanol 70%). Caffeic acid was included as a reference compound. After this drug preincubation, 5 μ L of calcium ionophore A23187 was added in DMSO to give a final concentration of 1 μ M for a further 10 min of incubation. The cells were pelleted by centrifugation at 400 g for 10 min at 4°C, and the supernatants were used to measure the release of MPO. Control tubes contained cells incubated only with 5 μ L of calcium ionophore A23187 for measuring maximum enzyme release.
MPO release was determined by measuring the rate of oxidation of o-dianisidine in a microplate assay (CitationBradley et al., 1982). The following reagents were added to the wells of a 96-well plate in the order stated: 50 μ L supernatant, 50 μ L phosphate buffer (pH 6.0) containing 0.5% HTAB, 50 μ L of 0.68 mg/mL o-dianisidine in distilled water and 50 μ L freshly prepared 0.003% H2O2 to start the reaction. The optical density (OD) at 450 nM was read immediately and thereafter at 5 min intervals with intermittent shaking. The amount of enzyme, expressed as enzymatic activity (mU), in the samples was obtained by comparison of the rate of reaction with that in wells containing supernatants from the control group (only treated with A23187).
Any direct inhibitory effect of the extract on MPO enzyme activity was assessed by adding 50 μ L of freeze-thaw disrupted cells supernanant (as enzyme source), adding 50 μ L of an appropriate dilution of the extract or their vehicle and proceeding with the assay by adding o-dianisidine, detergent buffer and H2O2 as above.
Scavenging of hydrogen peroxide
The scavenging of hydrogen peroxide was measured by adding 100 μ L H2O2 1 mM and 900 μ L buffer NaCl/Tris HCl, pH 7.4, to 10 μ L of the stock solution of the compounds, in triplicate. After incubating the mixture for 30 min at room temperature, 50 μ L of a guaiacol solution (0.2% v/v in distilled water), and 10 μ L of horseradish peroxidase (1 mg dissolved in 200 μ L of the pH 7.4 buffer) was added. After a further 10 min, 0.2 mL aliquots of the samples were transferred to a 96-well plate and the OD450 values were read in a microplate reader.
Statistical analysis
Data from 8–10 experiments were pooled and expressed as arithmetic means ± SEM. The data were evaluated using an ANOVA test followed by Tukey's test for paired data and the non-parametric Mann-Whitney U-test (UI determination). P values were considered significant at P < 0.05.
Results
Effects of diclofenac and P. carpunya on gastric mucosa. Macro- and microscopic appearance
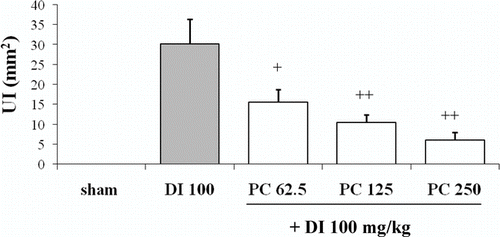
As shown in , diclofenac caused important damage on the glandular mucosa (30.2 ± 6.2 mm2). In contrast, pretreatment with P. carpunya 62.5, 125, and 250 mg/kg decreased the ulcerated area to 15.6 ± 3.1, 10.5 ± 1.9, and 6.02 ± 2.0 mm2, respectively (P < 0.01 and P < 0.05 versus diclofenac group).
Figure 1. Effects of administration of different doses of the dry residue from ethanol extract of P. carpunya on gastric damage induced by diclofenac treatment. +, P < 0.5; + +, P < 0.01 versus diclofenac group.
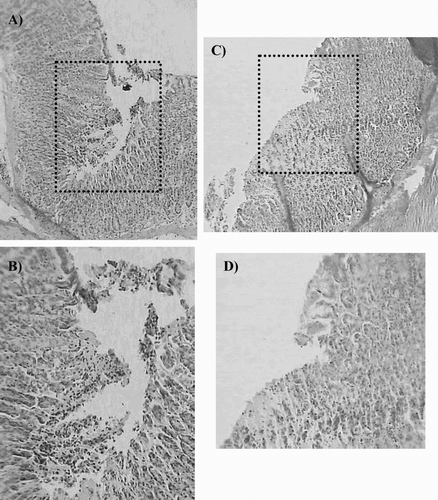
Histopathologic evaluation revealed that gastric mucosa of the control group had regular surface epithelial mucus cells and glandular cells (data not show). In the diclofenac group, the epithelium was ulcerated in most areas, with degeneration and dilatation of gastric glands ( and ). Besides an intense inflammatory reaction and local infiltration of leukocytes, diclofenac treatment caused significant epithelial damage with detached surface epithelial cells, hence on the surface of the stomach there were prominent hollow spaces instead of mucous cells. Pretreatment with the extract prevented the deep ulceration and necrosis induced by the NSAID ( and ).
Figure 2. Microscopic study (H/E section) of gastric mucosa of animals pretreated with dry residue from ethanol extract of P. carpunya before ulcer induction with diclofenac. (A and B), gastric lesion after diclofenac administration; (C and D), gastric mucosa of rats treated with P. carpunya (250 mg/kg) before diclofenac administration. Original magnification × 100 and × 200 respectively.
Effects of diclofenac and P. carpunya on MPO activity
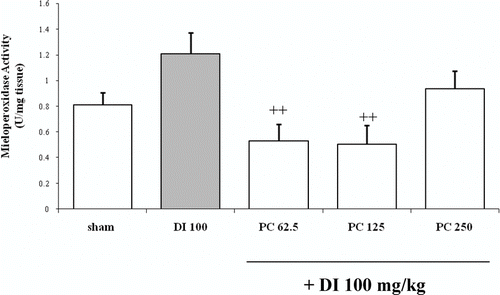
At the tested dose, diclofenac augmented MPO activity although it was not significant in comparison to sham group (1.207 ± 0.16 U/mg tissue and 0.81 ± 0.09 U/mg tissue, respectively) (). The two lowest doses of P. carpunya (62.5 and 125 mg/kg) decreased this enzymatic activity versus diclofenac group (0.53 ± 0.13 U/mg tissue and 0.50 ± 0.15 U/mg tissue respectively; P < 0.01). However, the highest dose of the plant, 250 mg/kg, did not inhibit the enzyme in a significant way (0.94 ± 0.14 U/mg).
Figure 3. Effects of administration of different doses of the dry residue from ethanol extract of P. carpunya on myeloperoxidase activity in gastric damage induced by diclofenac treatment. + +, P < 0.01 versus diclofenac group.
The in vitro results from the assays on rat peritoneal leukocytes are in accordance with those obtained in vivo. When rat peritoneal leukocytes are treated with A23187 an activation of the calcium-dependent secretion of the contents of the granules takes place. shows data of the MPO release, which occurred from the azurophilic granules of these cells (81.27 ± 14.11 mU). Both doses of P. carpunya, 25 and 50 μ g/mL, decreased significantly the MPO activity versus control treated with A23187 (24.47 ± 3.01 mU and 24.2 ± 8.0 mU, respectively; P < 0.01).
Figure 4. Effects of administration of different doses of the dry residue from ethanol extract of P. carpunya on myeloperoxidase activity on rat peritoneal leukocytes treated with calcium ionophore A23187. Caffeic acid was included as a reference compound. **, P < 0.01 versus A23187 group.
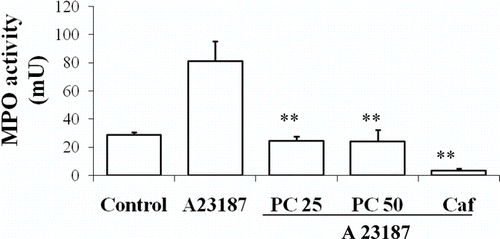
The subsequent experiment proved that the extract did not show a direct inhibition of the enzyme activity and, on the other hand, did not scavenge H2O2, which is the substrate for the enzymatic reaction (data not shown). Thus, it appears that the plant extract affects the pro-inflammatory secretory process by preventing MPO release from neutrophils.
Effects of diclofenac and P. carpunya on lipid peroxidation levels, superoxide dismutase and gluthatione peroxidase activity
Using TBARS in gastric mucosa as an index of lipid peroxidation, diclofenac treatment induced an important increase in the concentration of MDA above that of the sham group (sham 263.7 ± 18.04 nmol MDA/g tissue, diclofenac 439.17 ± 40.0 nmol MDA/g tissue, P < 0.01) (). Pretreatment with P. carpunya could not reverse the levels of lipoperoxides in gastric mucosa caused by diclofenac treatment because no significant difference could be observed in comparison with this group.
Figure 5. Effects of administration of different doses of the dry residue from ethanol extract of P. carpunya on lipid peroxidation, superoxide dismutase and glutathione peroxidase activity in gastric damage induced by diclofenac treatment. *, P < 0.05; and **, P < 0.01 versus sham group; +, P < 0.05 versus diclofenac group.
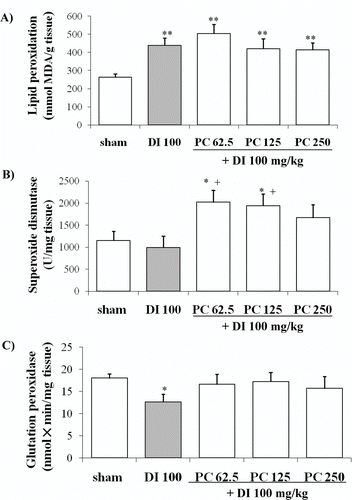
The values for SOD activity in the gastric mucosa of sham rats and diclofenac treatment were 1152.57 ± 204.41 U/mg tissue and 995.56 ± 258.33 U/mg tissue, respectively. Pretreatment with the extract at the doses of 62.5 and 125 mg/kg significantly increased the values in comparison to both control groups (2018.7 ± 275.15 U/mg tissue and 1935.9 ± 270.2 U/mg tissue, respectively, P < 0.05) ().
Diclofenac also caused an important depletion of GSH-Px activity (12.6 ± 1.8 nmol × min/mg tissue versus 17.96 ± 0.99 nmol × min/mg tissue of sham group, P < 0.05). The extract of P. carpunya reverted this enzymatic activity near to sham levels, but it was not statistically significant respect to the diclofenac group ().
Effects of diclofenac and P. carpunya on mucosal PGE2 content
Basal PGE2 content in gastric mucosa was 220.07 ± 80.75 pg/mg tissue and diclofenac caused a marked depletion (22.08 ± 4.30 pg/mg tissue; P < 0.05 vs. sham group). Pretreatment with P. carpunya extract at the three doses did not modify the PG content in comparison to diclofenac-treated animals ().
Discussion
The present study demonstrates for the first time that P. carpunya, a remedy used in folk medicine with anti-inflammatory and antiulcer properties, prevents NSAID-induced gastric mucosal injury. The aqueous suspension of the ethanol extract of the leaves of this species reduces the ulcerated area and improves the histological appearance of the gastric mucosa.
Over the last years evidence has accumulated on the implication of pro-inflammatory, oxidative and vascular mechanisms in the pathogenesis of NSAID-induced gastric lesions (CitationSánchez et al., 2002; CitationJiménez et al., 2004; CitationVillegas et al., 2004). Activated neutrophils that adhere to microvascular endothelium lead to the release of large amounts of bioactive substances, which are implicated in the pathophysiology of different inflammatory processes (CitationLefkowitz et al., 1999). The enzyme MPO, secreted by these cells, triggers the formation of reactive oxygen species with subsequent tissue injury, being an index of neutrophil infiltration (CitationKettle et al., 1997; CitationCarden & Granger, 2000). This is a potential source of oxygen free radicals, which are responsible for the tissue damage occurring in several inflammatory diseases (CitationKettle et al., 1997, CitationSullivan et al., 2000). Preventing the release of inflammatory mediators is a possible strategy to control the inflammation and this is the basic mechanism of action of a number of anti-inflammatory drugs. Pretreatment with the ethanol extract of P. carpunya prevented neutrophil infiltration in vivo induced by NSAID administration and inhibited the release of the proteolytic enzyme MPO by neutrophils stimulated with A23187. These in vitro results indicate that some components of the ethanol extract of this plant have an inhibitory influence on the secretory function of neutrophils, and this may also be a possible explanation for its anti-inflammatory activity shown in vivo.
This anti-inflammatory activity observed with P. carpunya has also been observed in other in vivo rat models like the carrageenan-induced paw edema, proving that the aqueous extract of this species significantly reduced the acute phase inflammation (Citationde las Heras et al., 1998).
Oxygen radical initiated lipid peroxidation and protein damage may account for the impaired cellular function and necrosis (CitationZimmerman & Granger, 1992). The MDA levels that indicate lipid peroxidation of the membranes were significantly increased after diclofenac treatment, which is related to tissue damage. In spite of the protective properties of P. carpunya, no changes in the TBA-reactive substances could be observed. These results are in agreement with the studies of Citationde las Heras et al. (1998) that showed a low activity of the ethanol extract of P. carpunya against lipid peroxidation in rat liver microsomes.
Preventive antioxidants such as SOD and catalase enzymes are the first line of defence against reactive oxygen species. GSH is an important constituent of intracellular protective mechanisms against a number of noxious stimuli and an important inhibitor of lipid peroxidation caused by free radicals (CitationHalliwell, 1995; CitationBafna & Balaraman, 2004). In the present study, the administration of the ethanol extract of P. carpunya exerted beneficial effects against diclofenac-induced gastric lesions by a potent increase in SOD activity, as well as by a recovery of the GSH-Px values. This indicates that the gastroprotection afforded by P. carpunya involves preservation of antioxidant enzymes activity in the mucosa exposed to damage.
Other recent experiments proved a significant in vitro free radical scavenging activity in the extract of leaves of Piper species (CitationBandyopadhyay et al., 2004) and similar results were obtained with other members of the same genus from Ecuador (CitationGuerrero et al., 2003).
Inhibition of PG synthesis is well recognized as the central mechanism by which gastrointestinal injury occurs (CitationGyires, 2005). In the stomach, protective PG synthesis is stimulated as a result of enhanced mucosal blood flow and stimulation of mucus and bicarbonate secretion (CitationHawkey, 2000). In this study, we observed that diclofenac treatment caused an important depletion of PGE2 in the stomach and the aqueous suspension of the extract was not able to reverse this situation.
As mentioned above, P. carpunya is a plant that has been viewed as potentially useful for the treatment of different pathologies as an anti-inflammatory and gastroprotective agent, although other uses have also been suggested (CitationBasten, 1981; CitationGupta, 1995). There are not many studies about this species and its chemical composition, but several works on the family Piperaceae report that the genus Piper has five phenolic amides, which possess significant antioxidant activity, even more effective than that of the naturally occurring antioxidant α -tocopherol (CitationNakatani et al., 1986).
In view of our results, we conclude that pretreatment of rats with aqueous suspension of the dry ethanol extract of P. carpunya protects the gastric mucosa against the injurious effect of NSAID administration by anti-inflammatory and antiradical mechanisms. A detailed study should be carried out in order to elucidate its phytochemical compounds and continue evaluating its gastroprotective effect in this relationship.
Acknowledgements
This work is included in a scientific cooperation programme of the Education and Science Ministry of Spain with Latin America (CYTED, Proyecto X.10).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- E Aizen, G Kagan, B Assy, R Iobel, Y Bershadsky, and A Gilhar. (2005). Effect of non-steroidal anti-inflammatory drugs on natural killer cell activity in patients with dementia. Isr Med Assoc J 7:78–81.
- C Alarcon de la Lastra, MJ Martin, C La Casa, and V Motilva. (1994). Antiulcerogenicity of the flavonoid fraction from Bidens aurea: Comparison with ranitidine and omeprazole. J Ethnopharmacol 42:161–168.
- PA Bafna, and R Balaraman. (2004). Anti-ulcer and antioxidant activity of DHC-1, a herbal formulation. J Ethnopharmacol 90:123–127.
- U Bandyopadhyay, K Biswas, A Sengupta, P Moitra, P Dutta, D Sarkar, P Debnath, CK Ganguly, and RK Banerjee. (2004). Clinical studies on the effect of Neem (Azadirachta indica) bark extract on gastric secretion and gastroduodenal ulcer. Life Sci 75:2867–2878.
- J Basten (1981): Las plantas medicinales de los Kallawasyas. La Paz, Proyecto Concern Bolivia, p.145.
- B Berenguer, LM Sánchez, A Quilez, M López-Barreiro, O de Haro, J Gálvez, and MJ Martín. (2006). Protective and antioxidant properties of Rhizophora mangle L. against NSAID-induced gastric ulcers. J Ethnopharmacol 113:194–200.
- JM Bjordal, AE Ljunggren, A Klovning, and L Slordal. (2004). Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: Meta-analysis of randomised placebo controlled trials. BMJ 329:1317.
- PP Bradley, DA Priebat, RD Christensen, and G Rothstein. (1982). Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78:206–209.
- SJ Bredie, H Wollersheim, FW Verheugt, and T Thien. (2003). Low-dose aspirin for primary prevention of cardiovascular disease. Semin Vasc Med 3:177–184.
- DL Carden, and DN Granger. (2000). . Pathophysiology of ischemia-reperfusion injury. J Pathol 190:255–266.
- B de las Heras, K Slowing, J Benedi, E Carretero, T Ortega, C Toledo, P Bermejo, SI Iglesia, MJ Abad, P Gomez-Serranillos, PA Liso, A Villar, and X Chiriboga. (1998). Anti-inflammatory and antioxidant activity of plants used in traditional medicine in Ecuador. J Ethnopharmacol 61:161–166.
- MB Grisham, JN Beniot, and DN Granger. (1990). Assessment of leukocyte in involvement during ischemia and reperfusion of intestine. Methods Enzymol 186:729–742.
- JK Grover, and SP Yadav. (2004). . Pharmacological actions and potential uses of Momordica charantia: A review. J Ethnopharmacol 93:123–132.
- RO Guerrero, SM Rivera, S Rivera, and LA Sueiro. (2003). Bioassay screening of Amazonian plants. PR Health Sci J 22:291–297.
- MP Gupta (1995): 270 Plantas Medicinales Iberoamericanas. Colombia, Convenio Andres Bello, Santa Fé de Bogotá: pp. 112, 126, 162.
- K Gyires. (2005). Gastric mucosal protection: From prostaglandins to gene-therapy. Curr Med Chem 12:203–215.
- B Halliwell. (1995). Antioxidant characterization: Methodology and mechanism. Biochem Pharmacol 49:1341–1348.
- CJ Hawkey. (2000). Nonsteroidal anti-inflammatory drug gastropathy. Gastroenterology 119:521–535.
- D Jiménez, MJ Martín, C Alarcon de la Lastra, L Bruseghini, A Esteras, JM Herrerías, and V Motilva. (2004). Role of L-arginine in ibuprofen-induced oxidative stressand neutrophil infiltration in gastric mucosa. Free Radic Res 38:903–911.
- AJ Kettle, CA Gedye, and CC Winterbourn. (1997). Mechanism of inactivation of myeloperoxidase by 4-aminobenzoic acid hydrochloride. Biochem J 21:503–508.
- C Lans, and G Brown. (1998). Observations on ethnoveterinary medicines in Trinidad and Tobago. Prev Vet Med 35:125–142.
- DL Lefkowitz, MP Gelderman, SR Fuhrmann, S Graham, JD Starnes, SS Lefkowitz, A Bollen, and N Moguilevsky. (1999). Neutrophilic myeloperoxidase-macrophage interactions perpetuate chronic inflammation associated with experimental arthritis. Clin Immunol 91:145–155.
- M Martin, C La Casa, V Motilva, A Lopez, and C Alarcon de la Lastra. (1996). Healing process induced by a flavonic fraction of Bidens aurea on chronic gastric lesion in rat. Role of angiogenesis and neutrophil inhibition. Z Naturforsch 51:570–577.
- CP McCord, and I Fridovich. (1969). Superoxide dismutase. J Biol Chem 244:6049–6055.
- MA Moroney, MJ Alcaraz, RA Forder, F Carey, and JRS Hoults. (1988). Selectivity of neutrophil 5-lipooxigenase and cyclo-oxygenase inhibition by an anti-inflammatory flavonoid glycosyde and related aglycone flavonoids. J Pharm Pharmacol 40:787–792.
- N Nakatani, R Inatani, H Ohta, and A Nishioka. (1986). Chemical constituents of peppers (Piper spp.) and application to food preservation: naturally occurring antioxidative compounds. Environ Health Perspect 67:135–142.
- H Ohkawa, N Ohishi, and K Yagi. (1979). Assay for lipid peroxide for animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358.
- C Sakamoto. (2003). NSAIDs caused gastric mucosal injury: With a special reference to COX-2. J Nippon Med Sch 70:5–11.
- S Sánchez, MJ Martín, P Ortiz, V Motilva, and C Alarcón de la Lastra. (2002). Effects of dipyrone on inflammatory infiltration and oxidative metabolism in gastric mucosa. Comparison with acetaminophen and diclofenac. Dig Dis Sci 47:1389–1398.
- W Sullivan, IJ Sarembock, and J Linden. (2000). The role of inflammation in vascular diseases. J Leukoc Biol 67:591–602.
- A Tapia, J Rodriguez, C Theoduloz, S Lopez, GE Feresin, and G Schmeda-Hirschmann. (2004). Free radical scavengers and antioxidants from Baccharis grisebachii. J Ethnopharmacol 95:155–161.
- I Villegas, C La Casa, C Alarcón de la Lastra, V Motilva, JM Herrerias, and MJ Martín. (2004). Mucosal damage induced by preferential COX-1 and COX-2 inhibitors: Role of prostaglandins and inflammatory response. Life Sci 74:873–884.
- HM Wang, and GY Zhang. (2005). Indomethacin suppresses growth of colon cancer via inhibition of angiogenesis in vivo. World J Gastroenterol 11:340–343.
- T Yoshikawa, Y Naito, A Kishi, T Kaneko, S Linuma, H Ichikawa, M Yasuda, S Takahashi, and M Kondo. (1993). Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut 34:732–737.
- BJ Zimmerman, and DN Granger. (1992). Reperfusion injury. Surg Clin North Am 72:65–83.