Abstract
The effect of aqueous and various organic extracts from the different parts of Areca catechu L. (Arecaceae) on oxidative DNA damage in human hepatocarcinoma HepG2 cells was investigated using single cell gel electrophoresis (comet) assay. Comparison of a series of organic extracts from the nut husk and different parts of this plant was performed. Incubation of methanol extract of Areca nut husk showed a dose-dependent inhibition of comet formation while other solvent extracts did not. Significant protection of H2O2-induced DNA damage was observed at 0.1% w/v concentration which was at the same level of protection by 1 μM butylated hydroxytoluene (BHT). The age of Areca catechu nut husk also played important role in chemical constituents exhibiting antioxidation since only two out of five different ages of husks displayed anti-oxidative effects in the comet assay. Among the different parts of this plant that were tested, the nut husk was the only consistent part that demonstrated antioxidant activity.
Introduction
Areca catechu L. (Arecaceae) or betel nut, chewed among older Thais and Asians, has been a cultural activity since ancient times. Arecoline, the major alkaloid present out of nine alkaloids found in the betel nut, possesses potent muscarinic action (CitationChu, 2001) and has now found an application in Alzheimer’s disease (CitationSullivan et al., 2000). The biological effects of betel quid chewing related to health hazards have been reviewed (CitationChu, 2002; CitationGupta & Ray, 2004; CitationJeng et al., 2001; CitationTrivedy et al., 2002; CitationWarnakulasuriya et al., 2002; CitationWinstock, 2002). These include neuronal dependence and oral health hazard leading to development of oral squamous cell carcinoma, including the preceding leukoplakia and submucous fibrosis. In addition, many investigators have examined the oxidative and genotoxic effects of betel nut extract or betel quid in several assay systems (CitationChang et al., 2002; CitationLiu et al., 1996; CitationJeng et al., 1994). On the contrary, others have investigated antioxidant properties. CitationLee et al. (2003) demonstrated that several herbal products containing Areca catechu extracts exhibited anti-oxidative activity in a DPPH free radical scavenging assay and inhibited lipid peroxidation. In Japan, CitationOhsugi et al. (1999) demonstrated that a methanol extract of betel nut seeds showed strong scavenging activity against superoxide anion radical (·O−2) in an electron spin resonance assay.
The comet assay or single cell gel electrophoresis (SCGE) assay is a rapid, sensitive and relatively simple method for detecting DNA damage at the level of individual cells. It combines the simplicity of biochemical techniques for detecting DNA single strand breaks (strand breaks and incomplete excision repair sites), alkali-labile sites, and cross-linking, with the single cell approach typical of cytogenetic assays. Other parts of this plant have not been studied for the antioxidant activity. Therefore, this study aimed to investigate the antioxidants of the rest of the betel plant. Several parts of the betel plant with particular focus on the nut husk were extracted using a series of organic solvents and distilled water, and subjected to the comet assay. The extent of oxidative DNA damage due to peroxide generated hydrogen peroxide was determined.
Materials and methods
Chemicals
BHT (CAS 128-37-0) was obtained from PC Dru, Thailand. Hydrogen peroxide (H2O2) (CAS 7722-84-1, lot #AF404317) was purchased from Ajax Finechem, Australia. Agarose (CAS 9012-36-6, lot #78K2170) was purchased from Gibco BRL, USA. DMSO (CAS 67-68-5, lot #33530) was purchased from Sigma-Aldrich Laborchemikalien, Germany. Tris (CAS 77-86-1, batch no. 57829) was purchased from Scharlau Chemie, Spain. All tissue culture biologicals were purchased from GibThai (Bangkok, Thailand). All other chemicals were AR grade purchased from Merck (Darmstadt, Germany).
Plant material and extraction
Different parts of Areca catechu (aerial root, ground root, leaves, crownshaft, and nut husk) were collected from Nakhon Pathom, Thailand, in October 2006. The voucher specimens were identified by Uthai Sotanaphun, Department of Pharmacognosy, Silpakorn University in Nakhon Pathom, Thailand and they were deposited in this department. Different parts of Areca catechu were washed with distilled water before hot air drying at 55°C for 72 h and then passed through sieve no. 60 and kept at 4°C before use. The 250 g dried nut husk of betel-nut tree was subsequently macerated in 1500 mL with various organic solvents. The sequential extraction for each solvent was 24 h, starting with petroleum ether, and followed by dichloromethane, ethyl acetate, methanol, and water. For other parts of the betel-nut plant, the extraction was done the same way, but only methanol was used. The overnight percolate was filtered through No. 1 Whatman® filter paper until clear. The extracts were then subjected to evaporation using a rotary evaporator (Büchi R205, Germany) until dry, and subsequently collected.
Cell culture
Human hepatocarcinoma cells (HepG2) were maintained in minimal essential medium (MEM) with 10% fetal bovine serum at 37°C in 5% CO2 humidified incubator. Cells were sub-cultured every 5–7 days in the absence of antibiotics.
Comet assay (single cell gel electrophoresis)
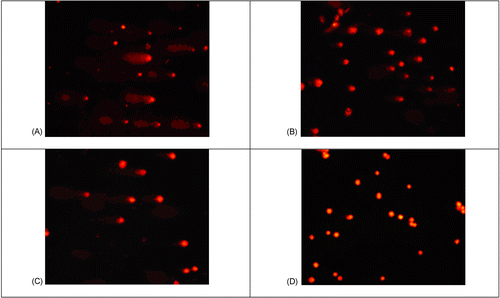
For single cell gel electrophoresis (SCGE) experiments, a 1 × 105 cell suspension with more than 99% pre-determined viability was subjected to oxidative DNA damage induced by incubation with 40 μM hydrogen peroxide for each reaction. Incubation was performed at 37°C in a microcentrifuge tube in the absence or presence of various concentrations of extracts of Areca catechu for 30 min. Comet slides for electrophoresis were made in-house by coating one side with 0.5% w/v normal melting agarose in water and left until the gel set. Briefly, each experiment consisted of 7 reactions; control, in which no hydrogen peroxide was added; reaction with 40 μM H2O2; H2O2 reaction with 4 concentrations of each extract, 0.1, 0.01, 0.005, and 0.001% v/v; and a positive control reaction by pre-incubation of the cells with 1 μM BHT in DMSO along with hydrogen peroxide. The 40 μM H2O2 treatment could induce the apparent apoptotic cell death which was characterized by tailing of the nucleus in the comet assay. The DMSO concentration in the final reaction volume was always kept below 2% v/v according to CitationUhl et al. (2000). Cells were incubated with designated Areca husk extracts (0.001, 0.005, 0.01 and 0.1%w/v) in 40 μL final reaction volume containing 1% low melting agarose (LMA) and later applied over the slide to allow solidification at 4°C, followed by another layer of 0.5% LMA. The slide was dipped into lysing solution at 4°C for at least 1 h, then transferred into an electrophoresis chamber containing electrophoresis buffer for at least 20 min before running. Electrophoresis was done at 4°C under 300 mA, 25 V for 40 min. After neutralization twice, the slide was immersed into absolute ethanol for 5 min and dried at room temperature. DNA was stained with ethidium bromide (20 μg/mL) and observed under a fluorescence microscope (Nikon Eclipse TE2000-S, excitation filter BP 543/10 nm, emission barrier filter 590 nm). Typical comet cells and results of inhibition are shown in .
Comet slide analysis
Antioxidant efficiency of extract could be determined by measuring the shortening of comet tails that migrated toward the anode during electrophoresis when DNA strands changed into fragments. The extent of DNA damage was then quantified using normalized tail length. Normalized tail length of the comet indicated the amount of damage, since longer tails indicated the strand breaks were frequent and the DNA was fragmented to several smaller molecules. One hundred comets on each slide were scored according to the length and relative intensity of fluorescence in the tail as compared to the head. Comets were selected at random from each slide. On-screen measurement of head and tail length was consistently performed.
Statistical analyses
The normalized tail length to head ratio was evaluated using one-way ANOVA for statistical analysis.
Results
Anti-oxidative effects of Areca catechu nut husk extracts
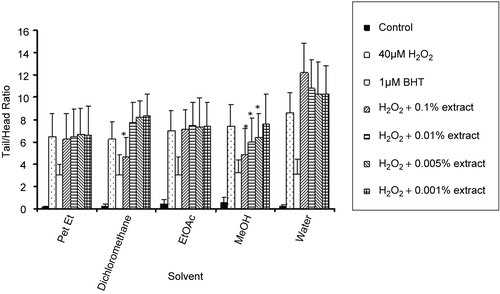
DNA damage was analyzed using the comet assay, a sensitive method for detecting DNA strand breaks in individual cells and a versatile tool that is highly efficacious in human bio-monitoring of natural compounds (CitationAruoma, 1999). A comet assay was set up by way of oxidative DNA damage induced with a free radical producer, H2O2, in order to screen for potential anti-oxidative compounds from betel-nut tree extracts. At first, the 8-month-old methanol extract of Areca nut husk was tested to look for optimal solvent in screening compounds of interest. As shown in , there was a consistent observation of anti-oxidative effect of the methanol extract of the betel nut husk when 0.1% w/v concentration was used. Cells treated with 40 μM H2O2 exhibited an average normalized tail length of 7.38 whereas cells treated with 1 μM BHT, which served as a positive control as an antioxidant, and with 0.1% w/v methanol extract, had values of 3.19 and 4.86 (p < 0.05), respectively. The experiment on husk methanol extract was repeated three times. Representative data are shown in .
Other organic as well as aqueous extracts of betel nut husk did not show significant reduction of tail DNA of the cells treated with various concentrations used in the assay. However, it was noticed in one experiment that 0.1% w/v of the methylene chloride extract was able to reduce DNA damage due to hydrogen peroxide (). Aqueous extract, however, significantly enhanced DNA damage.
Effect of age of Areca catechu nut husk on anti-oxidation
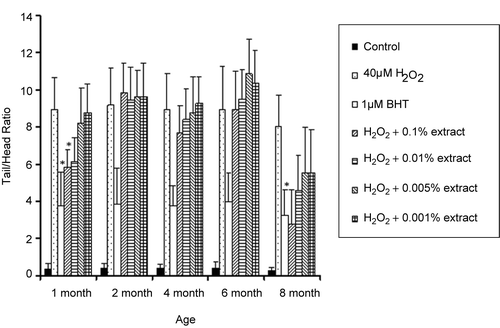
To determine the effect of Areca nut husk age on anti-oxidation, five other different ages of the nut husk extracted with methanol were tested. All of these husk extracts were tested with the same procedure as previously mentioned. In addition to the husk of 8-month-old Areca nut at 0.1% w/v extract, the results showed that the husk of 1-month-old Areca nut at 0.1 and 0.01% w/v could also inhibit hydrogen peroxide-induced DNA damage (normalized tail length of 5.84 and 6.12, respectively, p = 0.05), compared with the ratio from hydrogen peroxide (8.94) ().
Effect of methanol extracts of different parts of Areca catechu on antioxidation
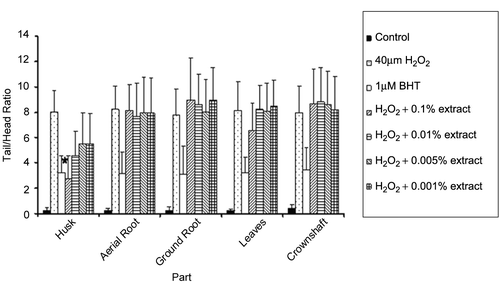
To obtain data indicating which parts of betel-nut tree could yield the highest degree of anti-oxidation, different parts of the plant, i.e., aerial root, ground root, leaves, and crownshaft, were extracted with methanol and tested for anti-oxidative properties. As shown in , 0.1% w/v methanol nut husk extract was the only sample that was active (p < 0.05) in this experiment. Other parts of betel-nut plant could not inhibit DNA fragmentation at any concentration tested.
Discussion
The single cell electrophoresis or comet assay for DNA damage is widely used for assessing antioxidant activity and DNA effects. This investigation demonstrated the antioxidative effects of Areca catechu nut husk extracts on an antioxidation assay using alkaline lysis single cell gel electrophoresis assay to detect DNA damage induced by hydrogen peroxide through generation of superoxide radical. From this assay, the extracts that exhibited antioxidant activity might reduce hydrogen peroxide-induced nuclear fragmentation or generate antioxidant enzyme, e.g., superoxide dismutase, catalase, glutathione peroxidase, to protect hydrogen peroxide-induced DNA apoptosis. Among various extracts, only the methanol extract of Areca catechu nut husk showed a significant inhibition of comet observed with HepG2 cells in a dose-dependent fashion. Furthermore, the 0.1% w/v methylene chloride extract exhibited an inhibition of comet formation. Similar results were noted with the methanol extract of Geum quellyon that could protect plasmid DNA from cleavage induced by. OH generated from UV-photolysis of hydrogen peroxide and NO (CitationRusso et al., 2005).
Assessment of different ages of Areca nut husk also revealed distinctive effects on oxidation. The methanol nut husk extract of 1- and 8-month-old Areca nut were found to have anti-oxidation activity while others did not. CitationGovindarajan and Mathew (1963) reported that the content of flavonoids from Areca nut changed over time. This indicated that the amount of compounds exhibiting antioxidation was changed during the ripening process. The amount of compounds exhibiting antioxidation from 1-month-old Areca nut husk was higher than those from the others. By visual observation, the color of Areca nut husk was changed from green to deep yellow at 8 months and, thus, flavonoids from this ripening process could promote the antioxidation. Methanol aerial root, ground root, crownshaft and leaf extracts of Areca catechu were devoid of antioxidation compared to the methanol nut husk extract. This antioxidative effect has never before been observed by any investigators in the herbal community. Nonetheless, these other parts were reported to have beneficial effects on various illnesses such as antipyretic effect and GI protection (CitationDuke, 1985). This preliminary result indicated that nut husk of this tree could be of interest for scientists in the antioxidation community since it was believed that inhibition of oxidative stress would lead to protection and/or treatment of diseases associated with aging. Therefore, the antioxidation constituents isolated from Areca catechu nut husk might contribute to improvement in age-related diseases.
Acknowledgments
This research work was kindly supported by the Faculty of Pharmacy, Silpakorn University. The authors would like to thank Dr. Juree Charoenteeraboon and Dr. Siripan Limsirichaikul for their help. Additionally, the authors express their gratitude to Ms. Sukuman Thammayat for her assistance. We appreciate Dr. Nalinee Poolsup and Dr. Wisit Tangkeangsirisin for their invaluable comments during the preparation of the manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aruoma OI (1999): Antioxidant action of plant foods: Use of oxidative DNA damage as a tool for studying antioxidant efficacy. Free Radical Res 30: 419–427.
- Chang MC, Ho YS, Lee JJ, Kok SH, Hahn LJ, Jeng JH (2002): Prevention of the Areca nut extract-induced unscheduled DNA synthesis of gingival keratinocytes by vitamin C and thiol compounds. Oral Oncol 38: 258–265.
- Chu NS (2001): Effects of betel chewing on the central and autonomic nervous systems. J Biomed Sci 8: 229–236.
- Chu NS (2002): Neurological aspects of Areca and betel chewing. Addict Biol 7: 111–114.
- Duke JA (1985): Handbook of Medicinal Herbs. Boca Raton, Florida, CRC Press, pp. 57–58.
- Govindarajan VS, Mathew AG (1963): Polyphenolic substances of Areca nut II. Changes during maturation and ripening. Phytochemistry 3: 657–665.
- Gupta PC, Ray CS (2004): Epidemiology of betel quid usage. Ann Acad Med Singapore 33: 31–36.
- Jeng JH, Chang MC, Hahn LJ (2001): Role of Areca nut in betel quid-associated chemical carcinogenesis: Current awareness and future perspectives. Oral Oncol 37: 477–492.
- Jeng JH, Jeng JH, Kuo ML, Hahn LJ, Kuo MY (1994): Genotoxic and non-genotoxic effects of betel quid ingredients on oral mucosal fibroblasts in vitro. J Dent Res 73: 1043–9.
- Lee SE, Hwang HJ, Ha JS, Jeong HS, Kim JH (2003): Screening of medicinal plant extracts for antioxidant activity. Life Sci 73: 167–179.
- Liu TY, Chen CL, Chi CW (1996): Oxidative damage to DNA induced by Areca nut extract. Mutat Res 367: 25–31.
- Ohsugi M, Fan W, Hase K, Xiong Q et al. (1999): Active-oxygen scavenging activity of traditional nourishing-tonic herbal medicines and active constituents of Rhodiola sacra. J Ethnopharmacol 67: 111–119.
- Russo A, Cardile V, Lombardo L, Vanella L, Vanella A, Garbarino A (2005): Antioxidant activity and antiproliferative action of methanolic extract of Geum quellyon sweet roots in human tumor cell lines. J Ethnopharmacol 100: 323–332.
- Sullivan RJ, Allen JS, Otto C, Tiobech J, Nero K (2000): Effects of chewing betel nut (Areca catechu) on the symptoms of people with schizophrenia in Palau, Micronesia. Br J Psychiatry 177: 174–178.
- Trivedy CR, Craig G, Warnakulasuriya S (2002): The oral health consequences of chewing Areca nut. Addict Biol 7: 115–125.
- Uhl M, Helma C, Knasmuller S (2000): Evaluation of the single cell gel electrophoresis assay with human hepatoma (Hep G2) cells. Mutat Res 468: 213–225.
- Warnakulasuriya S, Trivedy C, Peters TJ (2002): Areca nut use: An independent risk factor for oral cancer. Br Med J 324: 799–800.
- Winstock A (2002): Areca nut-abuse liability, dependence and public health. Addict Biol 7: 133–138.