Abstract
The alkaloids demissidine, dihydrosolacongestidine, solasodine and tomatidine were isolated and identified by spectroscopic methods and by comparison with literature data, from the aerial part of Solanum leucocarpum Dunal (Solanaceae) collected at the Regional Natural Park Ucumarí (Colombia). In order to examine topoisomerase I and II activities, the four isolated alkaloids were tested through a mechanism-based bioassay utilizing genetically engineered mutants of yeast Saccharomyces cerevisiae strains RS322N, R52Y and RS321. Dihydrosolacongestidine showed selective activity towards RS321 (249.8 μg/mL) strain rather than RS322N (1542.7 μg/mL), most probably indicating activity through inhibition of DNA topoisomerase II. In the same manner, tomatidine displayed activity through DNA topoisomerase II inhibition. Furthermore, solasodine showed DNA-damaging activity. In addition, the same isolated alkaloids were tested for antibacterial activity by using the agar well diffusion method. Solasodine and dihydrosolacongestidine showed MIC values of 62.5 and 125 μg/mL against Staphylococcus aureus, respectively. The isolation of these alkaloids and their biological activities are reported here for the first time for S. leucocarpum.
Introduction
The Solanaceae family is widely distributed in tropical zones around the world; it contains about 96 genera, which includes the large genus Solanum (CitationGriffin & Lin, 2000). Most of the species are herbaceous annuals or perennials, endemic to South America. The Solanaceae family is abundant and well represented in Colombian biodiversity. The Regional Natural Park Ucumarí (RNPU) is the richest, with 59 species reported. S. leucocarpum (Solanaceae) is a shrub that grows up to 5 m in height and from 100 to 2400 m above sea level in the Amazonian and Andean Colombian regions (CitationGaleano & Bernal, 1993).
From an ecological point of view, the steroidal glycoalkaloids are considered defense allelochemicals which have effects against a great number of pathogens and predators (CitationFukuhara et al., 2004). Concerning the commercial scope, solasodine has an advantage over other glycoalkaloids, being used as a raw material in the industrial synthesis of pharmaceutical steroids, such as progestagens, androgens, estrogens, norsteroids, and diuretic spirolactones (CitationWeissenberg, 2001; CitationOkršlar et al., 2002). In addition, steroidal glycoalkaloids display a wide range of in vitro biological activities such as antitumor, cytotoxicity against a variety of cancer cell lines (CitationNakamura et al., 1996), and anti-Herpes activity (CitationIkeda et al., 2003). Furthermore, they can act as teratogenic, embryotoxic, antifungal (CitationWeissenberg, 2001), DNA topoisomerase I and II inhibitors (CitationKim et al., 1996), leishmanicidal (CitationChan-Bacab & Peña-Rodriguez, 2001) and molluscicidal (CitationWanyonyi et al., 2002).
The study of this Solanaceae species was the result of several important considerations: the extensive use of Solanaceae plants in folk medicine, the therapeutic effect shown by the steroidal alkaloids from this family, the accelerated rainforest destruction, as well as the great Colombian flora biodiversity. Another important reason was the fact that, in a preliminary screening, the crude dichloromethane and methanol extracts from S. leucocarpum showed antibacterial and DNA damaging activities among 70 species studied. The combination of all these facts prompted us to isolate, characterize and to test the biological activities of four steroidal alkaloids obtained from the methanol extracts of S. leucocarpum collected at the Regional Natural Park Ucumarí (RNPU).
Materials and methods
General experimental procedures
Silica gel 60 (0.0400-0.063 and 0.063-0.200 mm) for flash and normal column chromatography, as well as silica gel 60 F254 and RP-18 F254 S for normal and reverse phase TLC were purchased from Merck (Darmstadt, Germany). In addition, RP-18 for reverse phase column chromatography was purchased from Aldrich (Milwaukee, WI). Analytical grade solvents were purchased from Mallinckrodt (Phillipsburg, NJ). Nystatin was from Sigma (St. Louis, MO). For the microbiological assays, yeast extract peptone dextrose (YEPD), Muller Hinton II agar (MH-II-A), and brain heart infusion agar (BHI) were purchased from Becton-Dickinson (Sparks, MD).
1H and 13C-NMR spectra were recorded on a Bruker Avance 400 spectrometer at 400 and 75 MHz, respectively. Deuterated chloroform or pyridine were used as solvents for the NMR experiments, chemical shifts were recorded in parts per million (ppm), using TMS as internal standard. For the high mass accuracy measurement samples were analyzed on a GC-MS spectrometer 6890 Hewlett Packard (Santa Clara, CA) coupled to a selective 5973 Hewlett Packard detector, equipped with the system HP-MS Chemstation (version B 0100). The melting points were taken on a B-540 Büchi instrument (Flawil, Switzerland).
Plant material
The aerial part of S. leucocarpum was collected at RNPU (Risaralda, Colombia) in November 2003 and classified by F.J. Roldán. A voucher specimen (FJR 3926) was deposited at the University of Antioquia Herbarium (Medellín, Colombia).
Extraction and isolation of alkaloids from S. leucocarpum
The collected plant materials were oven-dried at 50°C, ground to a fine powder and extracted 48 h by maceration three times successively with n-hexane, dichloromethane, and methanol, at room temperature. Then, the different extracts were concentrated at reduced pressure to dryness and stored at 210°C until analyzed.
A portion (50 g) of the methanol extract from S. leucocarpum was dissolved in water and extracted three times with n-hexane to degrease the hydro-alcoholic (H-A 1) extract. Then, the H-A 1 extract was treated three times with chloroform, which after vacuum concentration afforded 8.52 g (CHCl3 1). The H-A 1 phase was extracted three times with n-BuOH saturated with water to afford 10.5 g of n-BuOH extract which was solubilized in 350 mL of the mixture water:MeOH (7.5:2.5); then, the solution was made basic to pH 9 with concentrated ammonium hydroxide, and extracted three times with portions of 250 mL of chloroform. The chloroform phases were combined and concentrated to dryness to afford 24.24 g (CHCl3 2). After that, the latter chloroform extract was solubilized in MeOH and alkalinized to pH 11.75 with concentrated ammonium hydroxide to produce 8.42 g of a solid (S) and 2 g of the hydro-alcoholic phase (H-A 2). After solubilizing the solid S, in water-MeOH (7:3) and adjusting the pH to 2.5, extraction with chloroform afforded 300 mg (CHCl3 3).
Isolation of compounds 1, 2, 3 and 4
Seven hundred (700) mg of solid S after flash column chromatography (FCC) with the systems CHCl3-MeOH-H2O (78:20:2; 74:24:2) afforded fractions S-2 and S-3 with weights of 344 mg and 300 mg, respectively. FCC of fraction S-2 with the system CHCl3-MeOH-Me2CO-NH4OH(Conc) (74:14:14:2) yielded 22 mg of compound 1. FCC of fraction S-3 with the latter system produced 26 mg of compound 2. Then, 206.6 mg of fraction CHCl3 3 were subjected to ODS column chromatography (ODS-CC) with the system Me2CO-MeOH (7:3) to render fraction CHCl3-3-2 (143 mg) which, after FCC with the system CHCl3-n-BuOH-EtOH-NH4OH(Conc) (80:8:10:2), furnished the pure compound 3. FCC of fraction H-A 1 with the systems CHCl3-MeOH-H2O (80:18:2; 78:20:2) produced 144 mg of fraction (H-A 1-3) which, after ODS-CC with the system MeCN-H2O (8:2), yielded the pure compound 4. For alkaloid visualization on TLC, Dragendorff’s reagent was used.
Mechanism-based yeast bioassay
The yeast mutant bioassay was carried out using the following genetically engineered Saccharomyces cerevisiae strains RS322N, R52Y and RS321. All strains were kindly donated by D.G.I. Kingston. Cultures were grown on YEPD broth and a portion of them was resuspended in 0.9% saline solution to obtain a 25% transmittance at 600 nm and worked out according to the agar well diffusion method described by CitationRíos et al. (1988). The isolated alkaloids were dissolved in 95% ethanol and tested at concentrations of 1000, 500, 250, 125. and 62.5 μg/mL. Nystatin at 30.2, 16, and 16.9 μg/mL was used as positive controls for S. cerevisiae strains RS322N, RS321, and R52Y, respectively. Then, the plates were incubated at 30°C for 36 h, and the inhibition zones were measured in millimeters. All determinations were performed in triplicate with two replicates. Activity was determined from dose-response curves and is reported as IC12 (μg/mL) values, which is the concentration required for a pure compound to produce an inhibition zone of 12 mm in diameter, around each 6 mm diameters, well (Gunatilaka & Kingston, 1988).
In this bioassay, an extract is considered active if it displays selective activity against one or more repair-deficient yeast. For instance, an agent that displays greater activity against RS321 than RS322N, with an IC12 less than one third between both yeast strains and, in general, an IC12 less than 2000, most probably mediates its inhibitory activity through topoisomerase II. Conversely, greater activity against RS322N implies an inhibitory DNA topoisomerase I mechanism (CitationZhou et al., 2000).
Antibacterial assays
The four isolated alkaloids were evaluated for antibacterial activities through the agar well diffusion assay (CitationRíos et al., 1988) against Bacillus subtilis (ATCC No. 21556), Escherichia coli (ATCC No. 9637), Klebsiella pneumoniae (ATCC No. 10031), Pseudomonas aeruginosa (ATCC No. 27853) and Staphylococcus aureus (ATCC No. 6538). The isolated compounds were tested at the same concentrations employed with the yeast mutant assay (1000-62.5 μg/mL). In this assay, as positive controls, cefotaxime at 1000 μg/mL for S. aureus, at 750 μg/mL for E. coli, K. pneumonia and P. aeruginosa, respectively, and at 500 μg/mL for B. subtilis were used.
The results for the antibacterial assay were recorded for each alkaloid tested by measuring (mm) the zones of growth inhibition surrounding each well. The tests were carried out in triplicate and the mean of the inhibition zones were calculated. The minimum inhibitory concentrations (MICs) were determined as the lowest concentration of the tested compounds that inhibited bacterial growth on the plate (CitationWardakhan & Louca, 2007).
Results and discussion
Spectroscopic interpretation of steroidal compounds isolated from S. leucocarpum
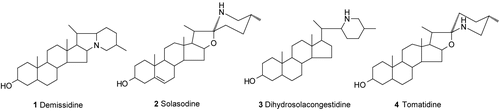
The structural elucidation of the isolated compounds was performed by using spectroscopic methods (MS, 1H and 13C NMR), and the results were compared with previously published data. Compound 1 was identified as demissidine (m.p. 164°C; m/z 400; molecular formula C27H45NO) (CitationRipperger, 1996; CitationRadeglia et al., 1977); 2 as solasodine (m.p. 205°C; m/z 414; molecular formula C27H43NO2) (CitationMahato et al., 1980); 3 as dihydrosolacongestidine (m.p. 230°C; m/z 413; molecular formula C27H44NO) (CitationSato et al., 1969); 4 as tomatidine (m.p. 167°C; m/z 416; molecular formula C27H45NO2) (CitationMahato et al., 1980; CitationUsubillaga et al., 1997; CitationLaurila, 2004). The chemical structures of the isolated compounds are shown in .
It is important to mention that compounds 1 and 2 have been isolated from other Solanaceae species belonging to the genus Solanum as are the cases from Solanum acaule (CitationRokka et al., 2005) and Solanum khasianum (CitationWeissenberg, 2001), respectively. Concerning compounds 3 and 4, they have been isolated from Solanum congestiflorum (CitationSato et al., 1969) and Lycopersicon esculentum (CitationWeissenberg, 2001), respectively. In addition, compounds 2 and 4 have been isolated from the ground berries of Solanum aculeastrum (CitationKoduru et al., 2007).
From a biosynthetic point of view, S. leucocarpum synthesized at least three different groups of steroidal alkaloids: spirosolanes, e.g., solasodine 2 and tomatidine 4, which are alike in their chemical structure but different biosynthetically; solanidanes, e.g., demissidine 1; and 22,26 epiminocholestanes, e.g., dihydrosolacongestidine 3. These findings are in concordance with CitationRoddick et al., (2001) since they consider Solanaceae as plants which are able to biosynthesize more than one type of steroidal glycoalkaloids, and this gives a clue about the hypothetical pathway for the formation of tomatidine, demissidine and solanidine from teinemine, and the conversion of solanidine to demissidine via a hydrogenase encoded enzyme, by a member of the genus Solanum (CitationRokka et al., 2005).
According to CitationCherkaoui et al., (2001), this work proves that, among the plants biosynthesizing steroidal glycoalkaloids, the main aglycone is solasodine, and S. leucocarpum is not the exception to this generalization. In addition, CitationRoddick et al., (2001) pointed out the role of glycoalkaloid in plants as defense molecules against insect pests and/or microbial pathogens. In fact, the action of these compounds against fungal diseases is well documented, although many plant secondary metabolites are known to have antibacterial activities (CitationAmir & Kumar, 2004).
Biological activities
The results for the DNA repair-deficient yeast mutant assay and the antibacterial activities for alkaloids 1, 2, 3 and 4 isolated from S. leucocarpum are reported in . It is important to mention there are no reports, on the secondary metabolites isolated from this species nor on the biological activities shown in this work.
Table 1. Results from the DNA repair-deficient yeast mutant and antibacterial assays for the steroidal alkaloids isolated from S. leucocarpum.
Compounds 1-4 were tested for their anticancer activity utilizing the mechanism-based bioassay employing genetically engineered yeast mutants where dihydrosolacongestidine and tomatidine exhibited DNA topoisomerase II inhibitory action, while solasodine showed DNA-damaging activity. These results are in agreement with those obtained from solasodine isolated from Solanum umbelliferum in the same bioassay (CitationKim et al., 1996). Furthermore, solasodine and tomatidine showed in vitro anticancer activity against HeLa, MCF7, and HT29 cancer cell lines, suggesting they may act on more than one pathway (CitationKoduru et al., 2007). In addition, experiments with S. cerevisiae showed that tomatidine inhibited the ergosterol biosynthesis pathway instead of being involved in membrane disruption (CitationSimons et al., 2006).
In regard to the antibacterial assay, it was found that solasodine (MIC 62.5 μg/mL) was more active than dihydrosolacongestidine (MIC 125 μg/mL) against pathogenic strain of Gram positive S. aureus; in contrast, the isolated steroidal alkaloids were less active against the other four microorganisms tested, showing weak activity in this assay. One possible explanation for this result is the fact that solasodine has the E ring intact. The E/F rings junction present in the spirosolane derivative and the unshared pair of electrons on the nitrogen atom in the F ring, favor the conditions for a strong solasodine activity in this type of assay. This result is in accordance with the suggestion that the presence of the nitrogen atom on the F ring is essential for the cytotoxic activity shown against HCT 116 (human colorectal carcinoma) cells (CitationQuan et al., 2002). Furthermore, both bioactive steroidal alkaloids can disrupt membranes since they can penetrate cell membranes by simple diffusion and reversibly binds to 3β-hydroxy sterols (CitationRokka et al., 2005). In relation to the steroidal glycoalkaloids interaction with sterols, the planar ring structure and the side-chain of sterols on position 24 turned out to be of major importance for membrane disrupting activity (CitationKeuken et al., 1995). Moreover, both alkaloids, once inside, can inhibit enzymes in the cholesterol pathway, blocking cell membrane biological activity and eventually producing death of the bacterium (CitationRokka et al., 2005).
The fact that solasodine displayed good antibacterial activity against S. aureus is of great value since multidrug resistant S. aureus is capable of surviving the effect of most antibiotics currently in use (CitationBozdag-Dündar et al., 2007). Therefore, solasodine could represent an attractive lead for the development of a new antibacterial agent.
Acknowledgments
The authors would like to thank the Universidad Tecnológica de Pereira, the Ministerio del Medio Ambiente y Desarrollo Territorial and CENICAFE for financial support of the project, and the Corporación Autonoma Regional de Risaralda (CARDER) for granting permission to access plant collection. We are grateful for the yeast strains used in this work which were kindly donated by D.G.I. Kingston (Virginia Polytechnic Institute and State University, VA, USA).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Amir M, Kumar S (2004): Possible industrial application of genus Solanum in the twenty-first century - A review. J Sci Ind Res 63: 116–124.
- Bozdag-Dündar O, özgen ö, Mentese A, Altanlar N, Atli O, Kendi E, Ertan R (2007): Synthesis and antimicrobial activity of some new thiazolyl thiazolidine-2,4-dione derivatives. Bioorg Med Chem 15: 6012–6017.
- Chan-Bacab MJ, Peña-Rodriguez LM (2001): Plant natural products with leishmanicidal activity. Nat Prod Rep 18: 674–688.
- Cherkaoui S, Bekkouche K, Christen P, Veuthey J-L (2001): Non-aqueous capillary electrophoresis with diode array and electrospray mass spectrometric detection for the analysis of selected steroidal in plant extracts. J Chromatogr A 922: 321–328.
- Fukuhara K, Shimizu K, Kubo I (2004): Arudonine, an allelophatic steroidal glycoalkaloid from the root bark of Solanum arundo Mattei. Phytochemistry 65: 1283–1286.
- Galeano MP, Bernal RG (1993): Guía de las plantas del Parque Regional Natural Ucumarí, Tomo I. CARDER. Pereira, Colombia, Editorial Gráficas Buda, p. 133.
- Griffin WJ, Lin GD (2000): Chemotaxonomy and geographical distribution of tropane alkaloids. Phytochemistry 53: 623–637.
- Gunatilaka AAL, Kingston DGI (1998): A DNA-damaging natural products with potential anticancer activity. In: ed., Atta-ur-Rahman, Studies in Natural Products Chemistry Vol. 20, Amsterdam, Elsevier, pp. 457–505.
- Ikeda T, Tsumagari H, Honbu T, Nohara T (2003): Cytotoxic activity of steroidal glycoalkaloids from Solanum plants. Biol Pharm Bull 26: 1198–1201.
- Keuken EAJ, de Vrije T, van den Boom C, de Waard P, Plasman HH, Thiel F, Chupin V, Jongen WMF, de Kruijff B (1995): Molecular bases of glycoalkaloids induced membrane disruption. Biochim Biophys Acta 1240: 216–228.
- Kim YC, Che QM, Gunatilaka AAL, Kington DGI (1996): Bioactive steroidal alkaloids from Solanum umbelliferum. J Nat Prod 59: 283–285.
- Koduru S, Grierson DS, van de Venter M, Afolayan AJ (2007): Anticancer activity of steroidal alkaloid isolated from Solanum aculeastrum. Pharm Biol 45: 613–618.
- Laurila J (2004): Interspecific hybrids of potato: Determination of glycoalkaloid aglycones and influence of bacterial infection. Thesis submitted to the Faculty of Agriculture and Forestry of the University of Helsinki, Yliopistopaino, Helsinki, p. 81.
- Mahato SB, Sahu NP, Ganguly AN, Kasai R, Tanaka O (1980): Steroidal alkaloids from Solanum khasianum: Apllication of 13C NMR spectroscopy to their structural elucidation. Phytochemistry 19: 2017–2020.
- Nakamura T, Komori C, Lee Y, Hashimoto F, Yahara S, Nohara T, Ejima A (1996): Cytotoxic activities of Solanum steroidal glycosides. Biol Pharm Bull 19: 564–566.
- Okršlar V, štrukelj B, Kreft S, Bohanec B, žel J (2002): Micropropagation and hairy root culture of Solanum laciniatum AIT. In Vitro Cell Dev Biol-Plant 38: 352–357.
- Quan H-J, Koyanagi J, Ohmori K, Uesato S, Tsuchido T, Saito S (2002): Preparations of heterospirostanols and their pharmacological activities. Eur J Med Chem 37: 659–669.
- Radeglia R, Adam G, Ripperger H (1977): 13C NMR spectroscopy of Solanum steroid alkaloids. Tetrahedron Lett 18: 903–906.
- Ríos JL, Recio MC, Villar A (1988): Screening methods for natural products with antimicrobial activity: A review of the literature. J Ethnopharmacol 23: 127–149.
- Ripperger H (1996): 22, 26-Epiminocholestane alkaloids with unusual (20R)-configurations from Solanum species. Phytochemistry 41: 1629–1631.
- Roddick JC, Weissenberg M, Leonard AL (2001): Membrane disruption and enzyme inhibition by naturally occurring and modified chacotriose-containing Solanum steroidal glycoalkaloids. Phytochemistry 56: 603–610.
- Rokka VM, Laurila J, Tauriainen A, Laakso I, Larkka J, Metzler M, Pietila L (2005): Glycoalkaloid aglycone accumulations associated with infection by Clavibacter michiganensis ssp. sepedonicus in potato species Solanum acaule and Solanum tuberosum and their interspecific somatic hybrids. Plant Cell Rep 23: 683–691.
- Sato Y, Sato Y, Kaneko H, Bianchi E, Kataoka H (1969): Alkaloids from Solanum congestiglorum. J Org Chem 34: 1577–1582.
- Simons V, Morrissey JP, Latijnhouwers M, Csukai M, Cleaver A, Yarrow C, Osborun A (2006): Dual effects of plant steroidal alkaloids on Saccharomyces cerevisiae. Antimicrob Agents Chemother 50: 2732–2740.
- Usubillaga A, Aziz I, Tettmanzi MC, Waibel R, Achenbach H (1997): Steroidal alkaloids from Solanum sycophanta. Phytochemistry 44: 537–543.
- Wanyonyi AW, Chhabra SC, Mkoji G, Eilert U, Njue WM (2002): Bioactive steroidal alkaloid glycosides from Solanum aculeastrum. Phytochemistry 59: 79–84.
- Wardakhan WTW, Louca NA (2007): Synthesis of novel pyrazole, coumarin and pyridazine derivatives evaluated as potential antimicrobial and antifungal agents. J Chilean Chem Soc 52: 1145–1149.
- Weissenberg M (2001): Isolation of solasodine and other steroidal alkaloids and sapogenins by direct hydrolysis-extraction of Solanum plants or glycosides therefrom. Phytochemistry 58: 501–508.
- Zhou B, Johnson R, Mattern M, Wang X, Hecht S, Beck H, Ortiz A, Kingston DGI (2000): Isolation biochemical characterization of a new topoisomerase inhibitor from Ocotea leucoxylum. J Nat Prod 63: 217–221.