Abstract
A one-step purification of Senna alata (L.) Roxb. (Leguminosae) extract using silica gel vacuum chromatographic technique provided an anthraquinone high-yielding S. alata leaf extract. This method was capable of improving its anthraquinone content as well as antifungal activity against dermatophytes. The extract contained anthraquinone content up to 16.7% w/w, and exhibited antifungal activity against Trichophyton rubrum, T. mentagrophytes and Microsporum gypseum with minimum inhibitory concentration (MIC) values of 15.6, 62.5, and 250 μg/mL, respectively. Stability evaluations of the anthraquinone high-yielding extract in several conditions through a period of 4 months found that the extracts were stable either kept under light or protected from light. The extracts were also stable under 4 ± 2°C, 30 ± 2°C and an accelerated condition, at 45°C with 75% relative humidity. The aqueous solutions of the extracts, at pH 5.5 and 7, exhibited good stability. These results indicate that the anthraquinone high-yielding S. alata extract possessed satisfactory antifungal activity and stability for a further development of herbal medicines.
Introduction
Senna alata (L.) Roxb. or Cassia alata L. (Leguminosae) is a shrub that has been traditionally used for the treatment of dermatophyte infections (CitationFarnsworth & Bunyapraphatsara, 1992). The anthraquinones, including aloe-emodin, rhein, emodin and chrysophanol were demonstrated as the active antifungal agents (CitationAgarwal et al., 1976, Citation2000; CitationZhou et al., 2006). Recently, poor quantity of S. alata leaves, due to the content of anthraquinones being lower than the standard value in the monograph, has been a major problem in the production of the herbal medicine from S. alata (CitationPanichayupakaranant & Intaraksa, 2003). Although an extraction method of S. alata leaf has been established, an extract with low anthraquinone content (1.67 %w/w) was still obtained. To improve the potency of the antifungal activity of S. alata leaf extract, the anthraquinone content of the extract should be increased. In addition, the interfering compounds in the extract, such as chlorophylls, should be excluded from the extract in order to improve the physical appearance and stability of the antifungal cream. As part of our interest in a purification method that can improve the anthraquinone content in S. alata leaf extract, chromatographic methods including silica gel vacuum chromatography and anion exchange chromatography were examined. Stability of the extract was also studied in order to get useful information for future studies on development of herbal medicines from the extract.
Materials and methods
Plant material
S. alata leaves were collected from Songkhla Province, Thailand, in November 2006. The voucher specimen (specimen no. SKP 097.1 03 01 01) was identified by Pharkphoom Panichayupakaranant and deposited at the herbarium of the Faculty of Pharmaceutical Sciences, Prince of Songkla University, Thailand. The leaves were dried at 50°C for 12 h in a hot air oven and were reduced to powder using a grinder and sieve no. 45.
Preparation of S. alata leaf extract
Dried plant powders (500 g) were successively extracted with methanol containing 5% (v/v) hydrochloric acid, 5% (w/v) ferric chloride and 15% (v/v) water (3.5 L × 2) under reflux conditions for 1 h. The pooled extracts were dried in vacuo. The extract was then partitioned between ethyl acetate and water. The ethyl acetate phase was dried and subjected to further isolation by either anion exchange chromatography or silica gel vacuum chromatography.
Isolation by anion exchange chromatography
The anion exchange resin (Amberlite® IRA-67, Sigma, USA) was treated with methanol and loaded into a column (10 × 126 cm). The column was washed twice with water and methanol, respectively. The ethyl acetate extract (25 g) was dissolved in methanol and loaded into the column with a flow rate of 5 mL/min. The column was eluted with methanol until chlorophylls were completely washed out. The anthraquinones were then eluted with 10% acetic acid in methanol. The pooled anthraquinone fractions were dried in vacuo.
Isolated by silica gel vacuum chromatography
A sintered glass column (13 cm in diameter) was packed with silica gel approximately 6 cm high. The ethyl acetate fraction (25 g), which pre-adsorbed on the silica gel, was loaded as a thin layer on the surface of column. The column was eluted with a mixture of hexane and ethyl acetate (9:1) (500 mL) with the aid of a vacuum pump. The pooled fractions of anthraquinones were then dried in vacuo.
Quantitative analysis of anthraquinones
HPLC conditions and calibration curves
HPLC analysis was carried out using Agilent 1100 series equipped with Agilent 1100 series Photodiode-array detector (PDA) and autosampler. Data analysis was performed using Agilent 3D ChemStation software (Agilent, USA). Separation was achieved isocratically at 25°C on a 150 mm × 4.6 mm i.d. TSK-gel ODS-80Tm column. The mobile phase consisted of methanol 2%, aqueous acetic acid 70:30 v/v, and was pumped at a flow rate of 1 mL/min. The injection volume was 20 μl. The quantitation wavelength was set at 254 nm. The calibration curves were established from the standards aloe-emodin and emodin at the concentration between 12.5–200 μg/mL. The linear equations of Y = 8397.7X – 1.34 (r2 = 0.9999) and Y = 4800.9X – 7.71 (r2 = 1.0000) correspond to aloe-emodin and emodin, respectively.
Sample preparation
The extracts were accurately weighed to 5 mg and dissolved in 10 mL methanol. The solutions were filtered through 0.45 μm membrane filter and subjected to the HPLC analysis.
In vitro antifungal activity assay
The compounds were sterilized by filtration through a membrane filter (0.45 μm) before testing. Clotrimazole and DMSO (1%) were used as positive and negative controls, respectively. Trichrophyton rubrum, T. mentagrophytes and Microsporum gypseum were grown in Sabouraud dextrose agar slant. The selected colonies were mixed with sterile physiological saline and the turbidity was adjusted by adding sterile physiological saline to McFarland turbidity standard 0.5 (106 colony forming units per mL).
MIC was determined using the agar dilution method (Lorian, 1996). The stock solution of the tested compounds was serial diluted with Sabouraud dextrose agar to give the final concentrations between 0.49 and 1000 μg/mL. Suspension of the test dermatophytes (2 μl) was added to each plate and incubated at 30°C for 3–5 days. The lowest concentration that did not show any growth of dermatophytes was taken as the MIC.
Stability evaluation
Effect of light on stability of the extract
The anthraquinone high-yielding S. alata leaf extracts were weighed to 100 mg and kept in well-sealed closed containers. The extracts were then stored at room temperature (30 ± 2°C) either protected from light or exposed to light for 4 months. An aliquot of each sample was taken at 0, 1, 2, 3, 4, 6, 8, 12, and 17 weeks and subjected to quantitative analysis of the anthraquinones using HPLC. The experiments were done in triplicate.
Effect of temperature on stability of the extract
The anthraquinone high-yielding S. alata leaf extracts were weighed to 100 mg and kept in well-sealed closed containers, protected from light. The extracts were then stored at 4 ± 2°C and room temperature (30 ± 2°C) for 4 months. Preparations for each temperature were done in triplicate. An aliquot of each sample was taken at 0, 1, 2, 3, 4, 6, 8, 12, and 17 weeks and subjected to quantitative analysis of the anthraquinones using HPLC. The experiments were done in triplicate.
Effect of pH on stability of the extract
The anthraquinone high-yielding S. alata leaf extracts were accurately weighed to 100 mg and dissolved in phosphate buffer solution to achieve pH values of 5.5, 7.0, and 8.0. The sample solutions were kept in well-closed containers, protected from light and stored at room temperature (30 ± 2°C) for 4 months. An aliquot of each sample was taken at 0, 1, 2, 3, 4, 6, 8, 12, and 17 weeks and subjected to quantitative analysis of the anthraquinones using HPLC. The experiments were done in triplicate.
Effect of accelerated conditions for stability of the extract
The anthraquinone high-yielding S. alata leaf extracts were weighed to 100 mg and kept in well-closed containers, protected from light. The extracts were then stored in a stability chamber at 45°C, 75% humidity for 4 months. An aliquot of each sample was taken at 0, 1, 2, 3, 4, 6, 8, 12, and 17 weeks and was subjected to quantitative analysis of the anthraquinones using HPLC. The experiments were done in triplicate.
Statistical analysis
Values are expressed as mean ± standard deviation (SD). Data were analyzed by Student’s t-test. The level of statistical significance was taken at p < 0.05.
Results and discussion
Preparation of the anthraquinone high-yielding S. alata extract
On the basis of HPLC analysis, the crude extract of S. alata leaves contained aloe-emodin and emodin as the major anthraquinones. The total anthraquinone in the crude extract was 1.13 ± 0.025% w/w. Chromatographic methods were used to concentrate the anthraquinone in the leaf extracts of S. alata as well as to diminish the other compounds. Two chromatographic methods, anion exchange and silica gel vacuum chromatography, were examined to improve the anthraquinone content in the leaf extract. After isolation by both methods the extracts still contained aloe-emodin and emodin as the major components (). The HPLC-chromatograms also showed that the polar interference compound was markedly excluded from the extract when using silica gel vacuum chromatographic method. Both methods were capable of increasing the total anthraquinone content in the extracts. However, the extract that was isolated by silica gel vacuum chromatography gave higher content of total anthraquinones than that isolated by anion exchange chromatography (). The silica gel vacuum chromatographic method increased total anthraquinone content in the extract by up to 15 times from the crude extract. In addition, isolation by silica gel vacuum chromatography was less time consuming than isolation by anion exchange chromatography. The results indicated that silica gel vacuum chromatography was a preferable method for improving the anthraquinone content in S. alata extract. This method produced the yellowish semisolid extract. The yield of the extract was 0.73 ± 0.09% w/w compared to the weight of dried leaf powder. The total anthraquinone content in the extracts was 16.22 ± 1.12% w/w (). The extracts used in further studies were standardized to the total anthraquinone content not less than 15% w/w.
Figure 1. HPLC-chromatograms of A) authentic anthraquinones, B) S. alata leaf extracts isolated by silica gel vacuum chromatography, and C) anion exchange chromatography.
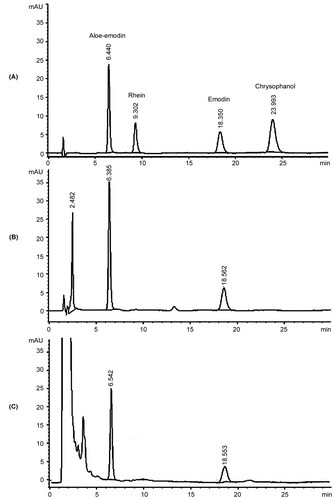
Table 1. Anthraquinone content in S. alata leaf extracts isolated by different methods.
Table 2. Anthraquinone content of the anthraquinone high-yielding S. alata extracts.
Antifungal activities of the anthraquinone high- yielding S. alata leaf extract
Evaluation of antifungal activities of the anthraquinone high-yielding S. alata leaf extract and the standard anthraquinones, aloe-emodin, rhein, emodin, chrysophanol against T. rubrum, T. mentagrophytes and M. gypseum found that the extract possessed antifungal activity against all tested dermatophytes with MIC values between 15.62–250 μg/mL (). The extract showed the highest antifungal activity against T. rubrum with a MIC value of 15.62 μg/mL. All tested dermatophytes were also completely inhibited by emodin and rhein at concentrations between 1.95–1000 and 31.25–1000 μg/mL, respectively. Although chrysophanol has been reported as the antifungal active compound (CitationIbrahim & Osman, 1995), the antifungal activity against all tested dermatophytes was not observed at concentrations up to 1,000 μg/mL. Among these tested compounds, aloe-emodin exhibited the strongest antifungal activity against T. rubrum with a MIC value of 0.98 μg/mL, but was not active against T. mentagrophytes and M. gypseum at concentrations up to 1,000 μg/mL. These results confirm a previous report on the two major antifungal constituents, aloe-emodin and emodin, of S. alata leaf extract against dermatophytes (Phongpaichit et al., 2004). Although the antifungal activity of the extract against T. rubrum was lower than that of aloe-emodin and emodin, the antifungal activities against T. mentagrophytes and M. gypseum were markedly higher than those of aloe-emodin and emodin. This may be due to the synergistic effect of these two active compounds. The results of these studies confirm the potential of the anthtraquinone high-yielding S. alata leaf extract as an antifungal active agent against dermatophytes.
Table 3. Antifungal activities of the anthraquinone high-yielding S. alata extract and the authentic anthraquinones.
Stability evaluation
Effect of light on the stability of the extract
The extracts were kept in the well-closed containers and stored either under fluorescent light or protected from light, at room 30 ± 2°C for a period of 4 months. The result demonstrated that under the light condition, the color of S. alata leaf extract gradually darkened. In contrast, the physical appearance of the extract kept in a container protected from light did not change through the period of 4 months. However, the anthraquinone content of the extract kept in both conditions did not decrease through a period of 4 months (). This finding suggests that the anthraquinone high-yielding S. alata leaf extract should be kept in a well-sealed closed container, protected from light, in order to stabilize the physical appearance.
Table 4. Anthraquinone content of the anthraquinone high-yielding S. alata leaf extracts stored under light and protected from light conditions.
Effect of temperature on the stability of the extract
The effect of temperature on the stability of the anthraquinone high-yielding S. alata leaf extract was examined under two temperatures, 4°C and 30°C, protected from light. The results showed that both tested temperatures did not affect either the physical appearance of the extracts or the anthraquinone content through the four-month period (). This implies that the anthraquinone high-yielding S. alata leaf extract is stable between temperatures of 4°C and 30°C at least through the period of 4 months.
Table 5. Anthraquinone content of the anthraquinone high-yielding S. alata leaf extracts stored under 4°C and 30°C.
Effect of accelerated condition on the stability of the extract
The accelerated stability test of the anthraquinone high-yielding S. alata leaf extract was carried out using a stability chamber at 45°C and 75% RH. The result demonstrated that the physical appearance as well as the anthraquinone content of the extract did not change even when stored under the accelerated condition through the period of 4 months (). This result implies that the anthraquinone high-yielding S. alata leaf extract is stable when kept in a well-closed container protected from light and stored at room temperature for at least two years.
Table 6. Anthraquinone content of the anthraquinone high-yielding S. alata leaf extracts stored under accelerated conditions.
Effect of pH on the stability of the extract
The acid-base stability evaluation of the anthraquinone high-yielding S. alata leaf extract in solution was determined at three different pH values: 5.5, 7, and 8. It was found that at pH 5.5 and 7, the anthraquinone content of the extract did not decrease through the period of four months (); neither the peak of any degradation product was observed in the HPLC-chromatograms. These results suggest that the anthraquinone high-yielding S. alata extract is stable under weak acidic and neutral pH through the period of 4 months. In contrast, at pH 8, decrements of aloe-emodin and emodin contents were observed at 17 and 4 weeks of storage, respectively. Emodin was markedly decreased after 4 weeks of storage. This result indicates that aloe-emodin and emodin are not stable in the alkaline solution. Thus, alkaline conditions should be avoided for further application of the anthraquinone high-yielding S. alata leaf extract.
The results from the stability tests indicate that the anthraquinone high-yielding S. alata leaf possesses satisfactory stability for further development of herbal medicines.
Table 7. Anthraquinone content of the anthraquinone high-yielding S. alata leaf extracts in solution at pH 5.5, 7, and 8.
Acknowledgements
The authors wish to thank the National Research Council of Thailand and the Graduate School, Prince of Songkla University, for support in the form of a research grant.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agarwal JS, Rastogi RP, Srivastava OP (1976): In vitro toxicity of constituents of Rumex maritimus Linn. to ringworm fungi. Curr Sci 45: 619–620.
- Agarwal SK, Singh SS, Verma S, Kumar S (2000): Antifungal activity of anthraquinone derivatives from Rheum emodi. J Ethnopharmacol 72: 43–46.
- Farnsworth NR, Bunyapraphatsara N (1992): Thai Medicinal Plant, Recommended for Primary Health Care System. Bangkok, Prachachon, pp. 90–93.
- Ibrahim D, Osman H (1995): Antimicrobial activity of Cassia alata from Malaysia. J Ethnopharmacol 45: 151–156.
- Lorian V (1996): Antibiotic in Laboratory Medicine 4th edn. Baltimore, Williams & Wilkins, pp. 28–49.
- Panichayupakaranant P, Intaraksa N (2003): Distribution of hydroxyanthracene derivatives in Cassia alata and the factors affecting the quality of the raw material. Songklanakarin J Sci Technol 25: 497–502.
- Phongpaichit S, Pujenjob N, Rukachaisirikul V, Ongsakul M (2004): Antifungal activity from leaf extracts of Cassia alata L., Cassia fistula L. and Cassia tora L. Songklanakarin J Sci Technol 26: 741–748.
- Zhou X, Song B, Jin L, Hu D, Diao C, Xu G, Zou Z, Yang S (2006): Isolation and inhibitory activity against ERK phosphorylation of hydroxyanthraquinones from rhubarb. Bioorg Med Chem Lett 16: 563–568.