Abstract
The present study investigated the antitumor effect and antioxidant role of the methanol extract of Oxystelma esculentum R. Br. (Asclepiadaceae) (MEOE) on tumor growth and the host survival time with mice. The antitumor and antioxidant potential of Oxystelma esculentum were studied against Ehrlich’s ascites carcinoma cell line (EAC) treated mice. MEOE was administered at doses of 200 and 400 mg/kg body weight (bw) once a day for 9 days after 24 h of tumor inoculation. Among the treated animals, six animals were sacrificed for biochemical and tumor analysis, and the remaining four groups were kept to study lifespan. On day 10, the parameters of tumor volume, packed cell volume, viable, and non-viable cell count were studied. Hematological and liver biochemical parameters, and antioxidant enzymes such as lipid peroxidation (LPO), glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), etc. were estimated. Decreases in tumor volume, packed cell volume, and viable cell count were observed in MEOE-treated mice when compared to EAC-treated mice. Treatment with MEOE at doses of 200 and 400 mg/kg increased the mean survival time to 29.66 ± 0.71 and 34.33 ± 2.34 days, compared with EAC-treated mice at 19.16 ± 1.13 days. The extract also decreased the body weight of the EAC-bearing mice. Hematological profiles indicated a decrease in white blood cells (WBC), an increase in red blood cells (RBC), and, thereby, Hemoglobin (Hb). MEOE restored all the parameters of hematological profiles to approximately normal. Treatment with MEOE decreased the levels of LPO and increased the levels of GSH, SOD, and CAT. These data indicate the methanol extract of Oxystelma esculentum exhibits significant antitumor activity, which might be due to the antioxidant effects on EAC bearing hosts.
Introduction
Cancer is a disease of misguided cells which have high potential of excess proliferation without apparent relation to the physiological demand of the process. It is the second largest single cause of death in both men and women, claiming over six million lives each year worldwide. In modern medicine, chemotherapy, radiotherapy, and surgery are the three major existing modes of treatment. Intervention with chemopreventive agents in the early stage of carcinogenesis is theoretically more rational than attempting to eradicate fully developed tumors with chemotherapeutic agents (CitationAnon, 2001).
Medicinal plants belong to the oldest known healthcare products that have been used by mankind all over the world in the form of traditional medicines. The World Health Organization (WHO) estimates that 80% of the world population relies on medicinal plants for primary health. World trade in medicinal plants and plant products thereof is estimated to be US $5 trillion by 2050 AD (CitationWilson, 1998). Free radicals have been implicated in the causation of several diseases such as cancer, diabetes, etc., and compounds that can scavenge free radicals have great potential in ameliorating these disease processes (CitationLollinger, 1981). Antioxidants thus play an important role to protect human body against damage by reactive oxygen species (CitationAjith & Janardhanan, 2003).
Plant-derived natural products such as flavonoids (CitationOsawa et al., 1990), terpenes (CitationGiulia et al., 1999) and alkaloids (CitationWitherup et al., 1990) have received considerable attention in recent years due to their diverse pharmacological properties including cytotoxic and cancer chemopreventive effects (CitationRoja & Heble, 1994). The target of much research has been on the discovery of natural and synthetic compounds that can be used in the prevention and/or treatment of cancer. Natural products of either plant or animal origin that exhibit antitumor activities have been discovered (CitationPezzuto, 1997).
Oxystelma esculentum R. Br. (Asclepiadaceae) is a perennial twining herb. It is distributed throughout the wild in the plains, on hedges and among bushes usually near water and lower hills of India, Ceylon, and Java (CitationGamble, 1957; CitationChopra et al., 1958). A decoction of the plant is used as a gargle in aphthous ulcerations of the mouth and in sore throat. The root is considered specific for jaundice, and milk sap is used as a wash for ulcers (CitationChopra et al., 1956; CitationNadkarni, 1954; CitationAnon., 1962). In Ayurveda, the plant is used as a diuretic, aphrodisiac, anthelmintic, for bronchitis, and useful for leucoderma; the fruit is expectorant, anthelmintic; the juice is used for gonorrhea, and for muscle pain (CitationKirthikar & Basu, 1935).
The present study was planned to investigate the in vivo antitumor activity of the methanol extract of aerial parts of Oxystelma esculentum against Ehrlich’s ascites carcinoma cell line in mice.
Materials and methods
Preparation of plant extract
The aerial plant material was collected in the month of January 2005 from riversides of Srirangapatnam, Near Mysore, Karnataka, India. The plant material was identified and authenticated by Prof. Revenna, Head of the Department of Botany, Kuvempu First Grade College, Channapatna, Karnataka, India (voucher no. DAKJU/02-2005) and the specimen was preserved in our laboratory for future reference. The dried aerial parts of the plant material (480 g) were powdered and initially defatted with petroleum ether (60-80°C) and further extracted with methanol in a Soxhlet apparatus for 72 h. The extract was then concentrated to dryness under reduced pressure and controlled temperature (50-60°C) to yield a dark blackish green semi-solid (yield 14.60% w/w), which was preserved in a refrigerator. The methanol extract was dissolved in distilled water and used for anticancer studies.
Chemicals
Trichloroacetic acid (TCA) was obtained from Merck (Mumbai, India). Thiobarbituric acid (TBA), nitro blue tetrazolium chloride (NBT) from Loba Chemie (Mumbai, India). 5,59-Dithio bis-2-nitro benzoic acid (DTNB), phenazonium methosulphate (PMS), nicotinamide adenine dinucleotide (NADH), Folin-Ciocalteau phenol and reduced glutathione (GSH) from Sisco Research Laboratory (Mumbai, India). Bovine serum albumin was purchased from Sigma Chemical Company (St. Louis, MO). 5-Fluorouracil (Fluracil) from Biochem pharmaceutical Industries (Mumbai, India). All the other reagents used were of analytical reagent grade.
Tumor cells
Ehrlich ascites carcinoma was developed by Loewenthal and Jahn from one of the several lines of Ehrlich carcinoma that arise from spontaneous epithelial tumors, probably of mammary gland origin. In the present study, the Ehrlich ascites carcinoma (EAC) cells were obtained from Chittaranjan National Cancer Research Center (Kolkata, India). The EAC cells were maintained by weekly intraperitoneal (i.p.) inoculation of 2 × 106 cells/mouse (CitationGothoskar & Ranadive, 1971).
Ethical clearance
The University Animal Ethical Committee approved experimental design performed in this study for the use of mice as an animal model for cancer activity.
Animals
Healthy male Swiss albino mice weighing 20 ± 2 g purchased from the Indian Institute of Chemical Biology, Kolkata were selected for the present investigation. The animal house was well ventilated and animals had 12 ± 1 h day and night schedule. The animals were housed in large spacious hygienic cages during the course of the experimental period. The animals were fed pellet feed supplied by M/s Hindustan Liver, (Kolkata, India) and water ad libitum.
Toxicity study
An acute toxicity study relating to the determination of LD50 was performed (CitationEcobichon, 1997).
Determination of antitumor activity
Male Swiss albino mice were divided into 5 groups (n = 10). All the groups were injected with EAC cells (0.2 ml of 2 × 106 cells/mouse) intraperitoneally (i.p.) except the normal group. This was taken as day zero. From the first day, normal saline 5 ml/kg/mouse/day were administered to normal and EAC control groups, groups 1 and 2, respectively, for 9 days i.p. Similarly, MEOE (200 and 400 mg/kg mouse/day) and standard drug, 5-fluorouracil (20 mg/kg) were administered in groups 3, 4, and 5, respectively. After administration of the last dose, followed by 18 h fasting, 6 mice from each group were sacrificed for the study of antitumor activity, hematological and liver biochemical parameters. The remaining animals in each of the groups were kept to determine the mean survival time (MST) of the tumor-bearing mice (CitationMazumder et al., 1997).
Tumor growth response
The antitumor potential of the processed extract was assessed by change in body weight, survival time, total ascites fluid volume, packed cell volume, tumor cell count, and hematological parameters. Viable and non-viable cells were counted by Trypan blue dye exclusion (0.4%) in a hemocytometer. Animal survival was recorded and expressed as median survival time (MST) in days, and percentage increase in lifespan (ILS) of processed extract-treated mice was calculated as follows:
An enhancement of lifespan by 25% or more was considered as an effective response (CitationGeran et al., 1972).
Hematological parameters
Hemoglobin content, red blood cell (RBC) and white blood cell counts (WBC) were measured from freely flowing tail vein blood (CitationD’Armour et al., 1965; CitationWintrobe et al., 1961). Differential leukocyte count of WBC was carried out from Leishman stained blood smears (CitationDacie & Lewis, 1958) of normal, EAC control, MEOE, and standard drug, 5-fluorouracil, treated groups, respectively.
Biochemical parameters
At 24 h after the last dose, and after 18 h fasting, blood was collected by puncturing retro-orbital plexus, the mice were sacrificed, liver was collected, and observed for pathological changes. Blood was used for the assay of biochemical parameters such as serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT) (CitationBergmeyar et al., 1978), serum alkaline phosphatase (SALP) (CitationKing, 1965), bilirubin (CitationMalloy & Evelyn, 1937), and total protein (CitationLowry et al., 1951).
Antioxidant assays
The liver was excised, rinsed in ice-cold normal saline followed by cold 0.15 M Tris HCl (pH 7.4), blotted dry, and weighed. A 10% w/v homogenate was prepared in 1.15% potassium chloride. A portion was utilized for the estimation of lipid peroxidation (CitationOhkawa et al., 1979) and another portion of the same, after precipitating proteins with TCA, was used for the estimation of glutathione (CitationEllman, 1959). The remaining homogenate was centrifuged at 1500 g for 15 min at 4°C. The supernatant thus obtained was used for the estimation of superoxide dismutase, catalase and protein (CitationKakkar et al., 1984; CitationAebi, 1974).
Statistical analysis
The experimental results were expressed as mean ± SEM. Data were assessed by the method of analysis of one way ANOVA followed by Dunnett’s t-test. p values of < 0.001 and 0.05 were considered as statistically significant.
Results
The present study revealed that MEOE showed significant antitumor and antioxidant activities in EAC- bearing mice. The activities of MEOE at the doses of 200 and 400 mg/kg on body weight, tumor volume, packed cell volume, tumor cell count (viable and non-viable cell), median survival time, %ILS, were studied ().
Table 1. Effect of the methanol extract of Oxystelma esculentum (MEOE) on body weight, tumor volume, packed cell volume, cell count, median survival time (MST) and percentage increase of lifespan (%ILS) in EAC-bearing mice.
Effect on mean survival time
In the EAC control group, the median survival time was 19.16 ± 1.13 days and it increased to 29.66 ± 0.71 and 34.33 ± 2.34 days for 200 and 400 mg/kg of MEOE treated groups, respectively. The standard drug 5-fluorouracil (20 mg/kg) treated groups showed 41.33 ± 1.74 days. MEOE treated groups, at both dose levels, reduced body weight, compared to the EAC treated control groups.
Effect on tumor growth
Treatment with MEOE at the doses of 200 and 400 mg/kg significantly (p < 0.001) reduced tumor volume and packed cell volume in a dose-dependent manner as compared to that of the EAC control group. Furthermore, percentage of non-viable tumor cell count at different doses of MEOE was effectively increased in a dose- dependent manner.
Effect on hematological profile
Hemoglobin content and RBC count in the EAC control group were significantly (p < 0.001) decreased as compared to the normal group. Treatment with MEOE at the dose of 200 and 400 mg/kg significantly (p < 0.05) increased the RBC count, and Hb content more or less normal levels. The total WBC count was found significantly increased in the EAC control group when compared with the normal group (p < 0.001). Administration of MEOE at doses of 200 and 400 mg/kg in EAC bearing mice significantly (p < 0.001) reduced the WBC count as compared with the EAC control. In a differential count of WBC, the presence of neutrophils increased, while the lymphocyte count decreased in the EAC control group. Treatment with MEOE at different doses changed these altered parameters approximately to the normal values ().
Table 2. Effect of the methanol extract of Oxystelma esculentum (MEOE) on hematological parameters in EAC-bearing mice.
Effect on biochemical parameters
Biochemical parameters like SGOT, SGPT, ALP and bilirubin in the EAC control group were significantly (p < 0.001) increased as compared to the normal group. Treatment with MEOE at doses of 200 and 400 mg/kg significantly (p < 0.001) decreased the SGOT, SGPT, ALP and bilirubin to approximately normal levels. The total protein was found significantly decreased in the EAC control group when compared with the normal group (p < 0.001). Administration of MEOE at doses of 200, and 400 mg/kg in EAC bearing mice significantly (p < 0.001) induced the total protein as compared with the EAC control ().
Table 3. Effect of methanol extract of Oxystelma esculentum (MEOE) on biochemical parameters in EAC-bearing mice.
Effect on lipid peroxidation and reduced glutathione
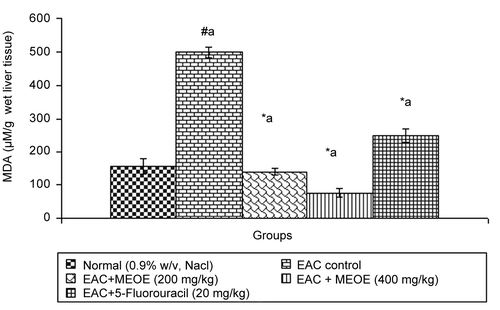
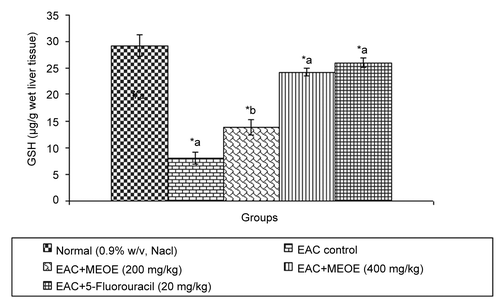
After administration of MEOE at 200 and 400 mg/kg and standard drug, 5-fluorouracil, to EAC bearing mice, the level of lipid peroxidation was significantly decreased (p < 0.001) by 138.99 ± 9.14, 76.00 ± 11.80, and 249.10 ± 20.17, respectively, in comparison to the EAC control group (). The administration of MEOE at different doses (200, and 400 mg/kg) and 5-fluorouracil to the EAC bearing mice increased GSH levels by 13.81 ± 1.43 (p < 0.05), 24.25 ± 0.85, and 26.07 ± 0.90 (p < 0.001), respectively, as compared with the EAC control group ().
Effect on antioxidant enzymes (SOD and CAT)
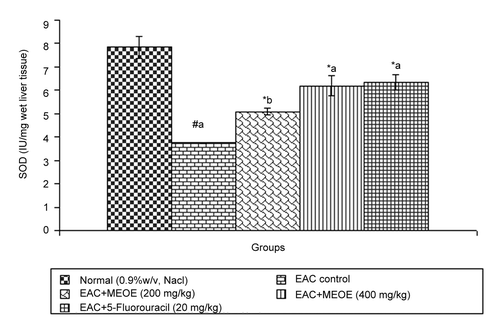
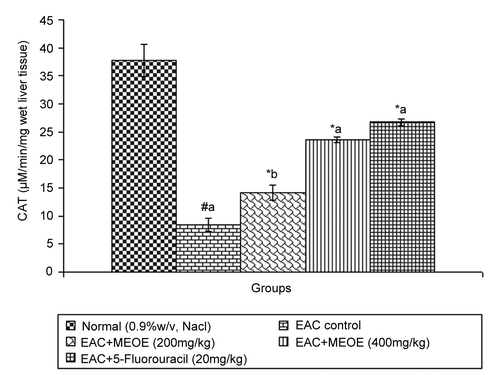
Administration of methanol extract of Oxystelma esculentum (MEOE) at different doses (200, and 400 mg/kg) and 5-fluorouracil increased the levels of SOD by 5.08 ± 0.15 (p < 0.05), 6.19 ± 0.43, and 6.35 ± 0.33 (p < 0.001), respectively, as compared to that of the EAC control group (). The catalase level at doses of 200 and 400 mg/kg and standard drug increased by 14.18 ± 1.37 (p < 0.05), 23.64 ± 0.51 and 26.76 ± 0.56 (p < 0.001), respectively, when compared to that of the EAC control ().
Discussion
The use of chemotherapeutic drugs in cancer therapy involves the risk of life-threatening host toxicity. The search therefore continues, to develop drugs which selectively act on tumor cells. The search for new antitumor agents from herbal plants has been extensively studied (CitationBabu et al., 1995). The present study was carried out to evaluate the toxicity, antitumor activity, lipid peroxidation and antioxidant potential of MEOE on EAC bearing mice. The MEOE treated animals at doses of 200 and 400 mg/kg inhibited the body weight, tumor volume, packed cell volume, tumor cell count, and also reverted the hematological parameters to approximately normal levels. Processed extract may have direct tumorocidal effect and thereby it may maintain the normal hematological profile. In EAC-bearing hosts, regular rapid increase in ascites tumor volume was observed.
Various plants have been used in cancer therapy as direct anticancer agents, chemotherapeutic agents, and radiosensitive or immunity enhancers (CitationPrasad & Giri, 1994). The ascites fluid is the direct nutritional source for tumor cells, and the faster increase in ascites fluid with tumor growth could possibly be a means to meet the nutritional requirements of tumor cells (CitationDahanukar et al., 2000). The reliable criteria for judging the value of any anticancer drug are the prolongation of lifespan of the animal (CitationClarkson & Burchneal, 1965) and control of WBC count in blood (CitationObeling & Guerin, 1954). The MEOE decreased the ascites fluid volume and thereby increased the percentage of lifespan. Viable tumor cell count was significantly inhibited in all the treated groups. The percentages of Trypan blue-positive dead tumor cells also increased in the treated groups as compared with the EAC control hosts. It may be concluded that MEOE, by decreasing the nutritional fluid volume and arresting the tumor growth, thereby increased the lifespan of EAC-bearing mice.
To evaluate whether MEOE treatment indirectly inhibited tumor cell growth, the effect of extract treatment was examined on the peritoneal exudate cells of normal mice. Normally, each mouse contains about 5 × 106 intraperitoneal cells, 50% of which are macrophages. MEOE treatment was found to enhance peritoneal cell counts. These results demonstrated the indirect effect of MEOE on EAC cells. This was probably mediated through enhancement and activation of macrophages or through cytokine product inside the peritoneal cavity (CitationKavimani & Manisenthilkumar, 2000) produced by MEOE treatment.
Usually in cancer chemotheraphy, the major problems encountered are of myelosuppression and anemia (Price & Greenfield, 1958; CitationHogland, 1982). The anemia encountered in tumor-bearing mice is mainly due to reduction in RBC or hemoglobin percentage, and this may occur either due to iron deficiency or due to hemolytic or myelopathic conditions (CitationFenninger & Mider, 1954). Treatment with MEOE restored the hemoglobin content, RBC and WBC cell count approximately to normal levels. This indicates that MEOE possesses protective action on the hemopoietic system.
Excessive production of free radicals results in oxidative stress, which leads to damage of macromolecules such as lipids, and can induce lipid peroxidation in vivo. Lipid peroxide formed in the primary site would be transferred through the circulation and provoke damage by propagating the process of lipid peroxidation. Increased lipid peroxidation would cause degeneration of tissues. Malondialdehyde (MDA), the end product of lipid peroxidation, was reported to be higher in carcinomatous tissues then in non-diseased organs (CitationYagi, 1991). Glutathione, a potent inhibitor of neoplastic process, plays an important role as an endogenous antioxidant system that is found particularly in high concentration in liver and is known to have key function in the protective process (CitationSinclair et al., 1990). MEOE reduced elevated levels of lipid peroxidation and increased the glutathione content in EAC bearing mice.
In the free radical scavenging system, superoxide dismutase (SOD) and catalase (CAT) are present in all oxygen metabolizing cells and their function is to provide a defense against the potentially damaging reactivities of superoxide and hydrogen peroxide (CitationRushmore & Picket, 1993). A decrease in SOD activity in EAC-bearing mice was reported to be due to loss of Mn2+ containing SOD activity in EAC cells and the loss of mitochondria, leading to a decrease in total SOD activity in the liver (CitationMarklund et al., 1982). The inhibition of SOD and CAT activities as a result of tumor growth was also reported (CitationSun et al., 1989). Similar findings were observed in the present study with EAC-bearing mice. The administration of MEOE at different doses increased SOD and CAT levels in a dose-dependent manner, which might be indicating the antioxidant and free radical scavenging property of MEOE.
Plant-derived extracts containing antioxidant principles showed cytotoxicity towards tumor cells (CitationJiau-Jian & Larry, 1977) and antitumor activity in experimental animals (CitationRuby et al., 1995). The antitumor activity of MEOE was accompanied with the increase of antioxidant status. The data revealed that the concentration of reduced glutathione, SOD and CAT were significantly increased when EAC-bearing mice were treated with MEOE. Conversely, the lipid peroxide concentration measured as MDA (malondialdehyde) was inhibited in the EAC-bearing mice on treatment with MEOE. This may be due to its role in the enhancement of the antioxidant system, at the same time being a free radical scavenger (CitationEl-khawaga et al., 2003). The free radical hypothesis supported that the antioxidant effectively inhibited the tumor, and observed investigations might be attributed to the antitumor and antioxidant principles present in the extract. The extract also restored the hepatic lipid peroxidation and free radical scavenging GSH as well as antioxidant enzymes such as SOD and CAT in tumor-bearing mice to approximately normal levels.
Furthermore, it may be concluded that the increase of lifespan of tumor-bearing mice by MEOE treatment is a positive result and supports the antitumor effect of MEOE. The results of the present study are encouraging, as the methanol extract has shown significant prolongation of lifespan, reduction in tumor volume, improvement in the hematological parameters of the hosts, and decreased the lipid peroxidation, thereby augmenting the endogenous antioxidant enzymes in the liver. The above parameters are responsible for the antitumor and antioxidant activities of Oxystelma esculentum. Further research work is ongoing in our laboratory to isolate and identify the active phytoconstituents responsible for the antitumor and antioxidant activity.
Acknowledgments
D. Ashokkumar is grateful to AICTE, New Delhi, India for providing financial support to this work.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aebi H (1974): Catalase estimation. In: ed. Berg Meyer HV, ed., Methods of Enzymatic Analysis. New York, Verlag Chemie, pp. 673–684.
- Ajith TA, Janardhanan KK (2003): Cytotoxic and antitumor activities of a polypore macrofungus, Phellinus rimosus (Berk) Pilat. J Ethnopharmacol 84: 157–162.
- Anon. (1962): Wealth of India-Raw Material, Vol. 7, Publication and Information Directorate, Council of Scientific and Industrial Research, New Delhi, India, pp. 200–201.
- Anon. (2001): British Herbal Pharmacopoeia, 10th edn., West Yorks, British Herbal Medicine Association, West Yorks,UK. pp. 71–83.
- Babu TD, Kuttan G, Padikkala J (1995): Cytotoxic and antitumor properties of certain taxa of Umbelliferae with special reference to Centella asiatica (L.) Urban. J Ethnopharmacol 48: 53–57.
- Bergmeyar HU, Scelibe P, Wahlefeld AW (1978): Optimization of methods of aspirate aminotransferase and alanine aminotransferase. Clin Chem 24: 58–61.
- Chopra RN, Chopra IC, Handa KL, Kapur LD (1958): Indigenous Drugs of India, 2nd edn. Calcutta, India, UN Dhur & Sons, p. 598.
- Chopra RN, Nayar SL, Chopra IC (1956): Glossary of Indian Medicinal Plants. New Delhi, India, Council of Scientific and Industrial Research, p. 184.
- Clarkson D, Burchneal JH (1965): Preliminary screening of antineoplastic drugs. Prog Clin Cancer 1: 625–629.
- D’Armour FE, Blood FR, Belden DA (1965): The Manual for Laboratory Works in Mammalian Physiology, 3rd edn. Chicago, University of Chicago Press, pp. 4–6.
- Dacie JV, Lewis SM (1958): Practical Hematology, 2nd edn. London, Churchill, pp. 38–48.
- Dahanukar SA, Kulkarni RA, Rege NN (2000): Pharmacology of medicinal plants and natural products. Ind J Pharmacol 32: 581–585.
- Ecobichon DJ (1997): The Basis of Toxicity Testing New York, CRC Press, pp. 43–86.
- El-khawaga Om-Ali Y, Salem Tarek A, Elshal Mohamed F (2003): Protective role of Egyptian propolis against tumor in mice, Clin chimi Acta 338: 11–16.
- Ellman GL (1959): Tissue sulphydryl groups. Arch Biochem Biophys 82: 70–77.
- Fenninger LD, Mider G (1954): In: Advances in cancer research. ‘Some aspects of carcinogenesis’. Grenstein JP, Haddow A. eds., Advances in Cancer Research, Vol. 2. New York, Academic Press, p. 244.
- Gamble JS (1957): Flora of the Presidency of Madras, Vol. 2. Calcutta, India, Botanical survey of India, p. 586.
- Geran RI, Greenberg NH, Mac Donald MM, Schumacher AM, Abbot BJ (1972): Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother Rep 3: 1–103.
- Giulia DC, Nicola M, Angelo AI, Francesco C (1999): Life science flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci 65: 333–353.
- Gothoskar SV, Ranadive KJ (1971): Anticancer screening of SAN-AB: An extract of marking nut, Semicarpus anacardium. Ind J Expt Biol 9: 372–375.
- Hogland HC (1982): Hematological complications of cancer chemotheraphy. Sem Oncol 9: 95–102.
- Jiau-Jian L, Larry WO (1977): Over expression of manganese-containing superoxide dismutase confers resistance to the cytotoxicity of tumor necrosis factor α and/or hyperthermia. Cancer Res 57: 1991–1998.
- Kakkar P, Das B, Vishwanath PN (1984): A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21: 130–132.
- Kavimani S, Manisenthilkumar KT (2000): Effect of methanolic extract of Enicostemma littorale on Dalton’s ascitic lymphoma. J Ethnopharmacol 71: 349–352.
- ed. King J (1965): The hydrolases-acid and alanine phosphatase. In: Practical Clinical Enzymology London, Van Nostrand Company Ltd., pp.191–208.
- Kirthikar KR, Basu BD (1935): Indian Medicinal Plants, Vol. 2., 2nd edn. Delhi, India, M/S.Bishen Singh Mahendra Pal Singh, pp. 1604–1606.
- Lollinger J (1981): Free Radicals and Food Additives. Taylor and Francis, eds., London, p. 121.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RI (1951): Protein measurement with the Folin-phenol reagent. J Biol Chem 193: 265–272.
- Malloy HT, Evelyn KA (1937): The determination of bilurubin with the photometric colorimeter. J Biol Chem 119: 481–490.
- Marklund SL, Westman NG, Lundgren E, Roos G (1982): Copper-and zinc-containing superoxide dismutase, manganese–containing superoxide dismutase, catalase, and glutathione peroxidase in normal and neoplastic human cell lines and normal human tissues. Cancer Res 42: 1955–1961.
- Mazumder UK, Gupta M, Maiti S, Mukherjee D (1997): Antitumor activity of Hygrophila spinosa on Ehrlich ascites carcinoma and sarcoma-180 induced mice. Ind J Expt Biol 35: 473–477.
- Nadkarni KM (1954): Indian Materia Medica, Vol. 1. Bombay, India, Popular Prakashan, pp. 891–892.
- Obeling C, Guerin M (1954): The role of viruses in the production of cancer. In: eds. Grenstein JP, Haddow A, Advances in Cancer Research, Vol. 2. New York, Academic Press, pp. 406–410.
- Ohkawa H, Onishi, Yagi K (1979): Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Bio Chem 95: 351–358.
- Om-Ali YEl-khawaga, Tarek A Salem, Mohamed F Elshal (2003): Protective role of Egyptian propolis against tumor in mice. Clin Chimi Acta 338: 11–16.
- Osawa T, Kawakishi S, Namiki M (1990): Role of dietary antioxidants in protection against oxidative damage. In: Basic Life Sciences: Antimutagenesis and Anticarcinogenesis Mechanisms II. Kuroda Y, Shankel DM, Waters M D, eds. Vol 52, New york, Plenum Press, pp. 139–153.
- Pezzuto JM (1997): Plant derived anticancer agents. Biochem Pharmacol 53: 121–133.
- Prasad S, Giri A (1994): Antitumor effect of cisplatin against murine ascites Dalton’s lymphoma. Ind J Expt Biol 32: 155–162.
- Price VE, Greenfield RE (1958): Anemia in Cancer, In: Grenstein JP, Haddaw A, eds., Advances in Cancer Research, Vol. V. New York, Academic Press, pp. 199–200.
- Roja G, Heble MR (1994): The quinoline alkaloid camptothecin and 9-methoxy camptothecin from tissue cultures and mature trees of Nothapodytes foetida. Phytochemistry 36: 65–66.
- Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R (1995): Antitumor and antioxidant activity of natural curcuminoids. Cancer Lett 94: 783–9.
- Rushmore TH, Picket CB (1993): Glutathione S-transferase, structure, regulation, and therapeutic implication. J Biol Chem 268: 11475–478.
- Sinclair AJ, Barnett AH, Lunie J (1990): Free radical and antioxidant systems in health and disease. Br J Hosp Med 43: 334–44.
- Sun Y, Oberley LW, Elwell JH, Sierra Rivera E (1989): Antioxidant enzyme activities in normal and transformed mice liver cells. Int J Cancer 44: 1028–33.
- Wilson RL (1998): Free radicals and tissue damage, mechanistic evidence from radiation studies. In: Biochemical Mechanisms of Liver injury. New York, Academic Press, p. 123.
- Wintrobe MM, Lee GR, Boggs DR, Bithel TC, Athens JW, Foerster J (1961): Clinical Hematology, 5th edn. Philadelphia, Lea & Febiger, p. 326.
- Witherup KM, Look SA, Stasko MW, Ghiorzi TJ, Muschik GM, Cragg GM (1990): Taxus spp. Needles contain amounts of taxol comparable to the stem bark of Taxus brevifolia: Analysis and isolation. J Nat Prod 53: 1249–1255.
- Yagi K (1991): Lipid peroxides and human diseases. Chem Physiol Lip 45: 337–351.