Abstract
The bignoniaceous Arrabidaea chica (Bonpl.) B. Verl. (syn. Bignonia chica), Bignoniaceae, popularly known as carajuru, puca panga, chica or pariri is very common in the Amazon region. Leaves of this plant are widely used as anti-inflammatory and astringent agents as well as remedies for intestinal colic, sanguine diarrhea, leucorrhea, anemia and leukemia. The action of the extract from leaves and stems of Arrabidaea chica on hepatic energy metabolism was investigated in the perfused rat liver and isolated mitochondria. In isolated mitochondria the Arrabidaea chica extract (0.25-1.25 mg/ml) decreased the respiratory coefficient with the substrates β-hydroxybutyrate and succinate. The activities of succinate-oxidase and NADH-oxidase were inhibited and the ATPase of intact mitochondria was stimulated. The extract also inhibited the glucose 6-phosphatase of isolated microsomes. The cellular content of glucose 6-phosphate was increased, while the level of ATP was reduced. In perfused livers the extract (0.25-1.0 mg/ml) caused stimulation of oxygen consumption, inhibition of gluconeogenesis from lactate and pyruvate and reduction of glucose release from endogenous glycogen. The results of this investigation suggest that the inhibitory effect of the extract on hepatic glucose production is mainly related to its actions on the mitochondrial energy metabolism.
Introduction
The bignoniaceous Arrabidaea chica (Bonpl.) B. Verl. (syn. Bignonia chica), Bignoniaceae, with the common names carajuru, puca panga, chica or pariri, is a liana growing in tropical America (CitationZorn et al., 2001). Although Arrabidaea chica has a limited occurrence in the south of Brazil, Paraguay and northeast of Argentina, Arrabidaea chica is very common in the Amazon region and leaves of this plant have been widely used as anti-inflammatory and astringent agents as well as remedies for intestinal colic, sanguine diarrhea, leucorrhea, anemia, and leukemia (CitationTakemura et al., 1995). In former times Indians of the Rio Meta and the Orinoco prepared a red pigment from the leaves for tattoos. It was found that the red color was related to the 3-desoxyanthocyanidin named carajurin (6,7-dihydroxy-5,4′-dimethoxy-flavylium). A bioguided phytochemical study of Arrabidaea chica leaves showed that carajurin is responsible for the transcription factor NF-κB inhibition by which the anti-inflammatory activity of Arrabidaea chica preparations have been explained (CitationZorn et al., 2001). Additional studies of the leaves of Arrabidaea chica revealed the presence of anthocyanins, flavonoids, tannins and phytosterols in addition to the 7,4′-dihidroxy-5-methoxyflavone (CitationTakemura et al., 1995; CitationScogin, 1980). As mentioned before, preparations of the crude plant are used for treating several diseases, but biochemical and physiological studies have not yet been carried out. However, the presence of flavonoids in the extract from leaves of Arrabidaea chica strongly suggest that the extract may act on liver cell energy metabolism, because several flavonoids, like quercetin, can exert a variety of effects on liver metabolism (CitationGasparin et al., 2003a, Citation2003b). The present work was thus undertaken to investigate possible actions of the extract on glycogen and glucose metabolism in perfused rat livers and on the respiratory activity and several membrane-bound enzymatic activities in isolated mitochondria and microsomes.
Materials and methods
Plant material and extract preparation
Leaves and stems of Arrabidaea chica were collected in April 2004, near Colorado do Oeste (Rondônia, Brazil), and identified by O.A. Guimarães of the Botany Department at the Federal University of Paraná-UFPR, Brazil. A voucher specimen is deposited at the Botanic Department Herbarium of the State University of Maringá-UEM, Brazil, under registration number SP 11428. Leaves and stems of Arrabidaea chica (220.4 g) were air dried at room temperature, powdered and extracted by maceration with an ethanol-water mixture (8:2) according to the method described by CitationPrista et al. (1975). The hydroalcoholic extract was filtered, evaporated under reduced pressure and lyophilized yielding 83.35 g of crude extract (37.81%). The lyophilized extract of the leaves and stems of Arrabidaea chica was stored at −20°C until further use.
Isolation of mitochondria
Rat liver mitochondria were isolated by differential centrifugation in a mannitol-sucrose medium, according to CitationVoss et al. (1961). Freeze-thawing disrupted mitochondria were used for the assay of membrane-bound enzymes. Intact mitochondria were frozen in liquid nitrogen and thawed rapidly at 37°C. This procedure was repeated three times. The resulting disrupted mitochondria were maintained at 0-4°C for use. Protein contents were measured using the method of CitationLowry et al. (1951). The standard was bovine serum albumin.
Determination of oxygen consumption, ADP/O ratio and respiratory control ratio
Oxygen consumption by intact mitochondria was measured polarographically using an incubation medium containing 5 mM potassium phosphate, 10 mM Tris-HCl (pH 7.4), 0.2 mM EGTA, 10 mM potassium chloride, 250 mM mannitol and 50 mg% fatty acid-free bovine serum albumin. The following substances were added to the incubation medium when required: succinate (10 mM), β-hydroxybutyrate (10 mM) and ADP (125 μM). The ADP/O ratio was calculated according to CitationChance and Williams (1955) and it represents the amount of ADP added to the incubation system divided by the amount of oxygen consumed during the active phase of respiration (state III respiration). The respiratory control (RC) ratio is given by the rate of active respiration which follows ADP addition divided by the rate of respiration after ADP exhaustion (state IV respiration) (CitationChance & Williams, 1955). In this and in other experiments with isolated mitochondria, the extracts were added to the incubation medium in the concentration range between 0.25 to 1.25 mg/ml. After preincubation with mitochondria for a period of 2 min, the reactions were initiated by the addition of specific substrates.
ATPase activity
The ATPase activity was assayed by measuring phosphate release (CitationPullman et al., 1960; CitationCaparroz-Assef et al., 2001). For intact mitochondria the reaction medium contained: 200 mM sucrose, 10 mM Tris-HCl (pH 7.4), 50 mM KCl and, when required, 100 μM 2,4-dinitrophenol. When freeze-thawing disrupted mitochondria were used as the enzyme source, the medium contained: 20 mM Tris-HCl (pH 7.4). The reaction was started by the addition of 5 mM ATP and stopped, after 20 min of incubation at 37°C, by the addition of ice-cold 5% trichloroacetic acid. Phosphate was measured as described by CitationFiske and Subbarow (1925).
Membrane-bound enzymatic activities
Freeze-thawing disrupted mitochondria were used as the enzyme source for assaying membrane-bound enzymatic activities. NADH-oxidase and succinate-oxidase activities were assayed polarographically using a 20 mM Tris-HCl (pH 7.4) medium (CitationSinger, 1974; CitationCaparroz-Assef et al., 2001). A polarographic assay was also run with TMPD (N,N,N′,N′-tetramethyl-p-phenylenediamine) plus ascorbate as substrates. The reactions were started by the addition of 10 mM NADH, 10 mM succinate or 0.2 mM TMPD + 5 mM ascorbate.
Isolation of microsomes
Microsomes were isolated by differential centrifugation according to CitationMihara and Sato (1972). The pellet of the last 105,000 g centrifugation, containing the microsomal fraction, was suspended in cold medium (4°C) containing 150 mM KCl and 10 mM Tris (pH 7.4). The volume was adjusted to achieve a protein concentration of 30 mg ml−1. Protein content was measured using the method of CitationLowry et al. (1951). The standard was bovine serum albumin. The glucose 6-phosphatase activity was measured at 37°C in a medium containing 100 mM KCl, 20 mM Tris-HCl (pH 6.5), 10 mM glucose 6-phosphate and 0.2-0.3 mg microsomal protein. The medium was supplemented with 5 mM MgCl2 and 5 mM ATP to measure the ATPase activity. Phosphate release was measured according to the method of CitationFiske and Subbarow (1925).
Liver perfusion
Male Wistar rats (weighing 200-250 g) were used in all experiments. All experiments were done in accordance with the world-wide accepted ethical guidelines for animal experimentation. For the surgical procedure, the animals were anesthetized by i.p. injection of sodium pentobarbital (50 mg/kg). Hemoglobin-free, non-recirculating perfusion was undertaken as described elsewhere (CitationKelmer-Bracht et al., 1984; CitationBracht et al., 2003). The perfusion fluid was Krebs/Henseleit-bicarbonate buffer, pH 7.4 (CitationKrebs & Henseleit, 1932), saturated with an oxygen/carbon dioxide mixture (95/5%). The fluid was pumped through a temperature-regulated (37°C) membrane oxygenator prior to entering the liver via a cannula inserted in the portal vein. The perfusion flow was constant in each individual experiment and it was adjusted between 30 and 35 ml min−1, depending on the liver weight. Samples of the effluent perfusion fluid were collected at 2 min intervals and analyzed for their metabolite content. When glycogen catabolism was measured, the livers of fed rats were used in the experiments. Livers from 24 h fasted rats were used for the measurement of gluconeogenesis. The Arrabidaea chica extract was dissolved into the perfusion fluid. Lactate and pyruvate were measured by standard enzymatic methods, using lactate dehydrogenase (CitationGutmann & Wahlefeld, 1974; CitationCzok & Lamprecht, 1974). Glucose was measured by an enzymatic-colorimetric method using glucose-oxidase (CitationBergmeyer & Bernt, 1974). The oxygen concentration in the venous perfusate was monitored continuously employing a Teflon-shielded platinum electrode. Metabolic rates were calculated from the input-output differences and the total flow rates and were referred to the wet weight of the liver. The hepatic contents of ATP and glucose 6-phosphate were measured after freeze-clamping the perfused liver with liquid nitrogen. The freeze-clamped livers were extracted with perchloric acid. The extract was neutralized with K2CO3 and assayed by means of standard enzymatic procedures (CitationLamprecht & Trautschold, 1974; CitationLang & Michal, 1974).
Statistical analysis
The statistical significance of the differences between parameters was evaluated by means of Student’s t-test or Student-Newman-Keuls test. The latter was applied after submitting the data to variance analysis. The results are mentioned in the text as the p values; p < 0.05 was adopted as a criterion of significance.
Results
The effect of the extract of Arrabidaea chica on oxygen consumption by intact mitochondria was measured using NAD+-dependent (β-hydroxybutyrate) and FAD-dependent (succinate) substrates in the presence of exogenously added ADP (state III respiration) or after ADP exhaustion (state IV respiration). The extract of the Arrabidaea chica was added to the incubation medium for final concentrations between 0.25 and 1.25 mg/ml as shown in . The mitochondrial respiration driven by succinate in the presence of ADP (state III respiration) was decreased by the extract. Statistically, the decreases were significant only at the concentrations of 1.0 and 1.25 mg/ml of the extract (). With β-hydroxybutyrate as substrate (), state III respiration was not significantly affected up to the concentration of 1.25 mg/ml of the extract. At this highest concentration of the extract, state III respiration was decreased by 57% (p < 0.01). State IV respiration tended to increase with increasing extract concentrations, but statistical significance was lacking in the range up to 1.25 mg/ml.
Figure 1. Effects of the extract of Arrabidaea chica on the respiratory activity of isolated rat liver mitochondria driven by β-hydroxybutyrate (A) and succinate (B). Each data point is the mean ± SEM of 6 independent experiments. +p < 0.05, **p < 0.01, *p < 0.001, ANOVA with Newman-Keuls test.
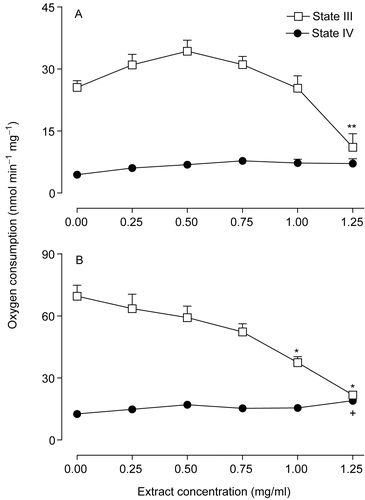
shows the effects of the Arrabidaea chica extract on the ADP/O ratios and the respiratory control ratios (RC). The respiratory control was progressively decreased by increasing concentrations of the extract with both substrates, β-hydroxybutyrate and succinate. With succinate as the substrate no respiratory control at all was found at the concentration of 1.25 mg/ml. At this concentration, evidently, no ADP/O ratio could be evaluated, but at lower extract concentrations the ADP/O ratio was not significantly decreased.
Table 1. The action of the Arrabidaea chica extract on mitochondrial respiration driven by β-hydroxybutyrate and succinate in the presence and absence of exogenously added ADP.
The effects of several concentrations of the Arrabidaea chica extract on NADH-oxidase activity, succinate-oxidase activity, and TMPD-ascorbate oxidation were measured in disrupted mitochondria and the mean values are summarized in . The extract promoted inhibition of both NADH-oxidase and succinate-oxidase activities in a dose-dependent manner. The mean concentration producing 50% inhibition (ID50) of succinate-oxidase activity was 0.79 ± 0.13 mg/ml. The maximal inhibition of the NADH-oxidase activity did not exceed 40%. TMPD-ascorbate oxidation was not significantly affected by the extract.
Figure 2. Effects of the extract of Arrabidaea chica on several membrane-bound enzymatic activities in rat liver mitochondria. (A) NADH-oxidase, succinate-oxidase activities and TMPD-ascorbate oxidation. (B) ATPase activity of coupled and uncoupled mitochondria. Each assay point represents the mean of 5-8 independent experiments and the bars are SEM. +p < 0.05, **p < 0.01, *p < 0.001, ANOVA with Newman-Keuls test.
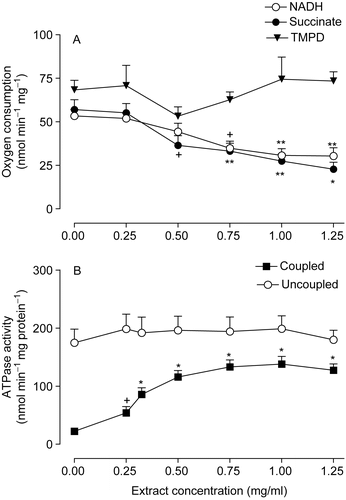
The effects of the Arrabidaea chica extract on the ATPase activity were measured in intact mitochondria either in the absence (coupled mitochondria) or in the presence of 2,4-dinitrophenol (uncoupled mitochondria), as shown in . The ATPase activity of coupled mitochondria was increased in a dose-dependent manner; maximal stimulation of 525% (p = 0.001) was achieved at an extract concentration of 1.0 mg/ml. When uncoupled mitochondria were used as the enzyme source, the ATPase activity was not significantly affected by the extract.
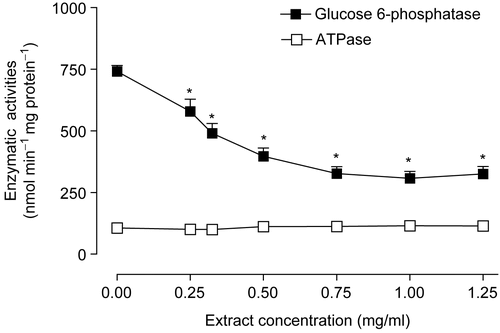
The substantial stimulation of the mitochondrial ATPase activity raised the question about the possible effects of the extract on the microsomal ATPase and glucose 6-phosphatase activities. The results illustrated in reveal that the microsomal ATPase activity was not affected. Glucose 6-phosphatase activity, however, was inhibited in a dose-dependent manner by the extract. The mean concentration producing 50% inhibition (ID50) of glucose 6-phosphatase activity was 0.69 ± 0.14 mg/ml.
Figure 3. Actions of the Arrabidaea chica extract on ATPase and glucose 6-phosphatase activities. Livers from fed rats were homogenized and subjected to differential centrifugation as described in Materials and methods. The microsomal fraction was used for glucose 6-phosphatase and ATPase assays. Initial rates were measured at various extract concentrations. The data points are the means of five determinations. Bars are standard errors of the mean, *p < 0.001, ANOVA with Newman-Keuls test.
Since the glucose 6-phosphatase activity was inhibited by the Arrabidaea chica extract, alterations in the glucose 6-phosphate levels can also be expected (CitationKelmer-Bracht et al., 2003). In the present work the levels of glucose 6-phosphate were measured before and after 30 min of 1.0 mg/ml extract infusion into perfused livers isolated from fed rats as shown in . The glucose 6-phosphate levels were increased by 65%. The levels of ATP which were measured under the same conditions, on the other hand, were reduced by 24%.
Table 2. Adenine nucleotide and glucose 6-phosphate contents of livers from fed rats in the presence and absence of the Arrabidaea chica extract.
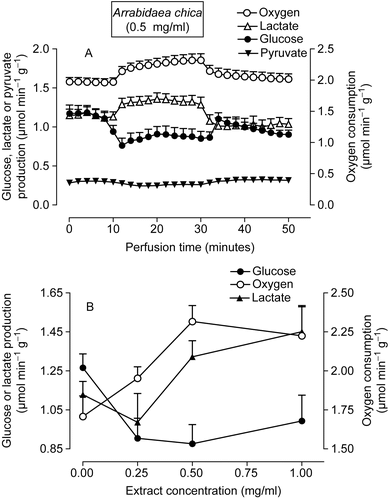
In order to verify whether the effects found in isolated mitochondria also manifest in intact cells, the actions of the Arrabidaea chica extract on oxygen consumption, glycogenolysis and glycolysis were investigated in the perfused liver of fed rats. Several experiments were undertaken in which the Arrabidaea chica extract was infused in the range between 0.25 and 1.0 mg/ml during 20 min. shows the results of representative experiments. These livers were perfused with substrate-free perfusion fluid, in an open system. Under these conditions, the livers release glucose, lactate and pyruvate as a result of glycogen catabolism (CitationScholz & Bücher, 1965). As shown in , the infusion of 0.5 mg/ml of Arrabidaea chica extract at 10 min of perfusion produced decreases in glucose release. At the end of the infusion, glucose release was reduced by 22% (p = 0.003) when compared with the rates measured before the infusion of the extract. The extract also increased oxygen consumption (18%, p = 0.002) and lactate production (18%, p = 0.009). When the infusion of the Arrabidaea chica was interrupted at 30 min, the metabolic fluxes tended to return slowly to the rates before infusion (basal rates). Experiments like those represented in were repeated with several extract concentrations and the mean values of glucose and lactate release and oxygen uptake at the end of the infusion period are summarized in . The maximal stimulation of oxygen uptake, as well as the maximal inhibition of glucose release were obtained with the concentration of 0.50 mg/ml.
Figure 4. Effects of the extract of Arrabidaea chica on metabolic fluxes in perfused livers isolated from fed rats. (A) Time course of the changes caused by the extract in glycogen catabolism and oxygen uptake. The extract (1.0 mg/ml) was infused at 10-30 min, as indicated by the horizontal bar. (B) Concentration dependence of the effects of the A. chica extract on glucose and lactate release and oxygen consumption. The experimental protocol was the same described for A. Each data point is the mean ± SEM of three experiments.
Glucose production from lactate and pyruvate was evaluated according to the protocol illustrated by in perfused livers from 24-h fasted rats. As expected, during the time period before lactate (2.0 mM) plus pyruvate (0.2 mM) infusion, glucose release was minimal because both the glycogen stores and the levels of endogenous gluconeogenic substrates are very low in livers from 24-h fasted rats (CitationWilliamson & Brosnan, 1974). The addition of the exogenously supplied gluconeogenic substrates immediately increased glucose production and oxygen consumption which stabilized after 20 min of infusion. The introduction of 1.0 mg/ml of Arrabidaea chica extract increased further the oxygen consumption (15%, p = 0.009). Glucose release, on the other hand, was diminished in the presence of the extract. At the end of the infusion, glucose release was reduced by 52% (p = 0.014) when compared with the rates measured before the infusion of the extract. These effects were reversible, that is, when the infusion of the Arrabidaea chica extract was interrupted at 50 min, the metabolic fluxes tended to return slowly to the rates before extract infusion.
Figure 5. Effects of the extract of Arrabidaea chica on metabolic fluxes in perfused livers isolated from fasted rats. (A) Time course of the changes caused by 1.0 mg/ml of extract in glucose production and oxygen uptake. Lactate (2 mM) and pyruvate (0.2 mM) were infused at 10–70 min and the extract at 30–50 min as indicated by the horizontal bars. (B) Concentration dependence of the effects of the A. chica extract on oxygen uptake and gluconeogenesis. The experimental protocol was the same described for A. Each data point is the mean ± SEM of four experiments.
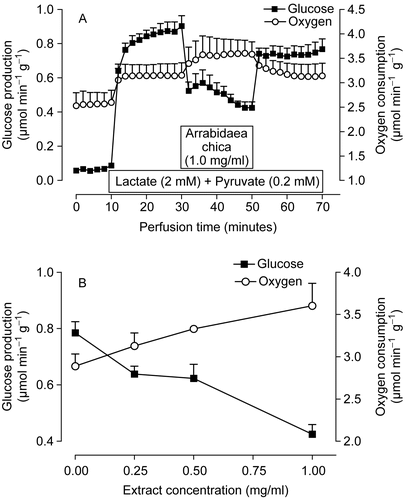
allows an evaluation of the changes caused by several concentrations of the Arrabidaea chica extract at the end of the infusion period on oxygen uptake and gluconeogenesis. The Arrabidaea chica extract was infused in the range of 0.25 to 1.0 mg/ml concentrations. Oxygen uptake was increased in a dose-dependent manner by the extract. Inhibition of gluconeogenesis was already evident at the concentration of 0.25 mg/ml, but increased considerably when the concentration of the extract was raised to 1.0 mg/ml.
Discussion
The results of the present work reveal that the extract of Arrabidaea chica is able to influence liver metabolism. The data obtained with isolated mitochondria showed that the extract inhibits the respiration coupled to ADP phosphorylation, reduces the respiratory control ratios and increases ATP hydrolysis of intact coupled mitochondria. These effects are characteristic of uncouplers such as 2,4-dinitrophenol (CitationHopfer et al., 1968; CitationHanstein, 1976) and non-steroidal anti-inflammatory drugs like piroxicam (CitationSalgueiro-Pagadigorria et al., 1996) and diclofenac (CitationPetrescu & Tarba, 1997). It is thus probable that the extract also acts as an uncoupler of oxidative phosphorylation. This conclusion is corroborated by the absence of stimulation of the mitochondrial ATPase activity of 2,4-dinitrophenol uncoupled mitochondria.
In addition to the uncoupling action the extract is also able to affect electron flow as indicated by the inhibition of NADH and succinate oxidation in disrupted mitochondria. The exact site of this action cannot be inferred from the data. The extract could be acting at any point on the electron transport chain between complex I and cytochrome c. The existence of more than one site of action is equally possible. Probably the inhibitory effect of the extract on the electron transport chain prevented stimulatory actions on state IV respiration which usually occur in the presence of mitochondrial uncouplers (CitationHopfer et al., 1968; CitationHanstein, 1976).
The data of rat liver perfusion provided evidence that the extract is also active on intact cells. It should be remarked that the intact liver differs from the system containing isolated mitochondria. While in an incubation system with isolated mitochondria the extract interacts directly with these organelles, in the case of the intact liver, the access of any compound to the mitochondria is influenced by several factors including plasma membrane transport, biotransformation, binding to intracellular components, etc.
The addition of the extract to the perfused livers caused activation of oxygen consumption either in livers from fed or fasted rats. Oxygen consumption stimulation could be the consequence of the uncoupling of oxidative phosphorylation which reduces the net rates of ATP production. Moreover, the lower rates of glucose production observed in the presence of the extract in livers from fasted rats could be, partly at least, due to depressed gluconeogenesis, since this pathway is strictly dependent on mitochondrial ATP production. On the other hand, an uncoupling action should equally produce stimulation of glycogenolysis and glycolysis (CitationKemmelmeier & Bracht, 1989; CitationNascimento et al., 1992; CitationConstantin et al., 1995; CitationSalgueiro-Pagadigorria et al., 1996). Lactate production was indeed slightly increased, suggesting some degree of glycolysis stimulation. However, glucose release from endogenous glycogen was not stimulated, actually it was decreased, whereas the opposite normally occurs when uncouplers are supplied to the liver cells (CitationKemmelmeier & Bracht, 1989; CitationNascimento et al., 1992; CitationConstantin et al., 1995; CitationSalgueiro-Pagadigorria et al., 1996). This observation suggests that the extract of Arrabidaea chica exerts other effects in addition to mitochondrial uncoupling. One such effect was actually detected in the present work, namely the glucose 6-phosphatase inhibition. Inhibition of this enzyme alone, however, should not in principle be responsible for the decreases in glucose release as indicated by experiments performed with livers from arthritic rats and other inhibitors of glucose 6-phosphatase. In arthritic rats, glucose production from glycerol is normal (CitationFedatto-Junior et al., 1999) in spite of a 60% reduced activity of glucose 6-phosphatase in these rats (CitationKelmer-Bracht et al., 2003). Moreover, inhibition of the enzyme by isosteviol does not avoid a several-fold stimulation of glucose release derived from glycogenolysis stimulation, which is also caused by the former compound (CitationIshii & Bracht, 1987). This occurs because inhibition of glucose 6-phosphatase shifts the steady-state glucose 6-phosphate concentration to higher levels, restoring the flux through the enzyme to levels reflecting the steady-state rates of glucose 6-phosphate production (CitationIshii & Bracht, 1987). An increase in the cellular glucose 6-phosphate concentration was indeed observed in the present work with the Arrabidaea chica extract. The same occurs in livers from arthritic rats (CitationKelmer-Bracht et al., 2003) and in perfused livers when isosteviol is infused (CitationIshii & Bracht, 1987). It could be, however, that glycogenolysis stimulation by the Arrabidaea chica extract was not as intense as necessary for supplying the glycolytic route with the amounts of glucose 6-phosphate required to maintain the energy status of the hepatocytes. In this case, competition between glucose 6-phosphatase and glycolysis would not allow the glucose 6-phosphate concentration to raise sufficiently so as to overcome inhibition. This interpretation needs, thus, the combination of three events: 1) increased glycolysis as a compensatory phenomenon for the diminished efficiency of mitochondrial energy transduction, 2) inhibition of glucose 6-phosphatase, and 3) some mechanism that does not allow glycogenolysis to be increased at the necessary levels. Events 1 and 2 were corroborated experimentally in the present work, but event 3 must be regarded here as a working hypothesis, still to be confirmed by future experiments. Future experiments must also provide an answer regarding the nature of the active substances implicated in the metabolic effects of the Arrabidaea chica extract. Only the testing of pure substances will allow definitive conclusions about the precise molecular mechanisms of action of Arrabidaea chica preparations.
Acknowledgements
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and from the Programa Nacional de Núcleos de Excelência (PRONEX).
Declarati on of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bergmeyer HU, Bernt E (1974): Determination of d-glucose with glucose oxidase and peroxidase. In: Bergmeyer HU, ed., Methods of Enzymatic Analysis. New York, Academic Press, pp. 1205–1215.
- Bracht A, Ishii-Iwamoto EL, Kelmer-Bracht AM (2003): O estudo do metabolismo no fígado em perfusão. In: Bracht A, Ishii-Iwamoto EL, eds., Métodos de Laboratório em Bioquímica. São Paulo, Editora Manole, pp. 275–288.
- Caparroz-Assef SM, Salgueiro-Pagadigorria CL, Amado CA, Bracht A, Kelmer-Bracht AM, Ishii-Iwamoto EL (2001): The uncoupling effect of the non-steroidal antiinflammatory drug nimesulide in liver mitochondria from adjuvant-induced arthritic rats. Cell Biochem Funct 19: 117–124.
- Chance B, Williams GR (1955): A simple and rapid assay of oxidative phosphorylation. Nature 175: 1120–1121.
- Constantin J, Ishii-Iwamoto EL, Suzuki-Kemmelmeier F, Yamamoto NS, Bracht A (1995): Bivascular liver perfusion in the anterograde and retrograde modes: Zonation of the response to inhibitors of oxidative phosphorylation. Cell Biochem Funct 13: 201–209.
- Czok R, Lamprecht W (1974): Pyruvate, phosphoenolpyruvate and d-glycerate 2-phosphate. In: Bergmeyer HU, ed., Methods of Enzymatic Analysis. New York, Academic Press, pp. 1446–1451.
- Fedatto-Junior Z, Ishii-Iwamoto EL, Bersani-Amado C, Vicentini GE, D’Urso-Panerari A, Bracht A, Kelmer-Bracht AM (1999): Gluconeogenesis in the liver of arthritic rats. Cell Biochem Function 17: 271–278.
- Fiske CH, Subbarow Y (1925): The colorimetric determination of phosphorus. J Biol Chem 66: 375–400.
- Gasparin FRS, Salgueiro-Pagadigorria CL, Ishii-Iwamoto EL, Bracht A, Constantin J (2003a): Action of quercetin on glycogen catabolism in the rat liver. Xenobiotica 33: 587–602.
- Gasparin FRS, Salgueiro-Pagadigorria CL, Ishii-Iwamoto EL, Bracht A, Constantin J (2003b): Actions of quercetin on gluconeogenesis and glycolysis in rat liver. Xenobiotica 33: 903–911.
- Gutmann I, Wahlefeld AW (1974): d(+) Lactate determination with lactate dehydrogenase and NAD+. In: Bergmeyer HU, ed., Methods of Enzymatic Analysis. New York, Academic Press, pp. 1464–1466.
- Hanstein W (1976): Uncoupling of oxidative phosphorylation. Biochim Biophys Acta 456: 129–148.
- Hopfer U, Lehninger AL, Thompson TE (1968): Protonic conductance across phospholipid bilayer membranes induced by uncouplers of oxidative phosphorylation. Proc Natl Acad Sci USA 59: 484–490.
- Ishii EL, Bracht A (1987): Glucose release by the liver under conditions of reduced activity of glucose 6-phosphatase. Braz J Med Biol Res 20: 837–843.
- Kelmer-Bracht AM, Ishii EL, Andrade PVM, Bracht A (1984): Construction of a liver perfusion apparatus for studies on metabolic regulation and mechanisms of drug action. Arq Biol Tecn 27: 419–438.
- Kelmer-Bracht AM, Barbosa-Santos CP, Ishii-Iwamoto EL, Broetto-Biazon AC, Bracht A (2003): Kinetic properties of the glucose 6-phosphatase of the liver from arthritic rats. Biochim Biophys Acta 1638: 50–56.
- Kemmelmeier FS, Bracht A (1989): Effects of the non-steroidal antiinflammatory mefenamic acid on energy metabolism in the perfused rat liver. Biochem Pharmacol 38: 823–830.
- Krebs HA, Henseleit H (1932): Untersuchungen tiber die Harnstoffbildung im Tier-körper. Z Physiol Chem 210: 33.
- Lamprecht W, Trautschold I (1974): Adenosine-5′-triphosphate. Determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU, ed., Methods of Enzymatic Analysis. New York, Academic Press, pp. 2101–2110.
- Lang G, Michal G (1974): d-glucose-6-phosphate and d-fructose-6-phosphate. In: ed. Bergmeyer HU, Methods of Enzymatic Analysis. New York, Academic Press, pp. 1238–1242.
- Lowry OH, Rosebrough NJ, Farr AC, Randal RLJ (1951): Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275.
- Mihara K, Sato R (1972): Partial purification of NADH-cytochrome b5 redutase from rabbit liver microsomes with detergents and its properties. J Biochem 71: 725–735.
- Nascimento EA, Kelmer-Bracht AM, Bracht A, Ishii-Iwamoto EL (1992): Activation of hepatic glycogenolysis by non-steroidal antiinflammatories is independent of Ca2+. Pharm Commun 3: 129–138.
- Petrescu I, Tarba C (1997): Uncoupling effects of diclofenac and aspirin in the perfused liver and isolated hepatic mitochondria of rat. Biochim Biophys Acta 1318: 385–404.
- Prista LN, Alves AC, Morgado R (1975): Técnica Farmacêutica e Farmácia Galenica. Lisboa, Editora Fundação Calouste Gulbenkian, pp. 1211.
- Pullman ME, Penefski HS, Datta A, Racker E (1960): Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphate. J Biol Chem 235: 3322–3329.
- Salgueiro-Pagadigorria CL, Kelmer-Bracht AM, Bracht A, Ishii-Iwamoto EL (1996): Effects of the nonsteroidal anti-inflammatory drug piroxicam on rat liver mitochondria. Comp Biochem Physiol 113C: 85–91.
- Scholz R, Bücher T (1965): Hemoglobin-free perfusion of rat liver. In: Chance B, Estabrook RW, Willianson JR, eds., Control of Energy Metabolism. New York, Academic Press, pp. 393–414.
- Scogin R (1980): Anthocyanins of the bignoniacea. Biochem Syst Ecol 8: 273–276.
- Singer TP (1974): Determination of activity of succinate, NADH, choline and glycerophosphate dehydrogenase. Methods Biochem Anal 22: 125–175.
- Takemura OS, Iinuma M, Tosa H, Miguel OG, Moreira E, Nozawa Y (1995): A flavone from leaves of Arrabidaea chica f. cuprea. Phytochemistry 38: 1299–1300.
- Voss DO, Campelo AP, Bacila M (1961): The respiratory chain and the oxidative phosphorylation of rat brain mitochondria. Biochem Biophys Res Commun 4: 48–51.
- Williamson DH, Brosnan JT (1974): Concentration of metabolites in animal tissues. In: Bergmeyer HU, ed., Methods of Enzymatic Analysis. New York, Academic Press, pp. 2266–2302.
- Zorn B, Garcia-Piñeres AJ, Castro V, Murillo R, Mora G, Merfort I (2001): 3-Desoxyantocyanidins from Arrabidaea chica. Phytochemistry 56: 831–835.