Abstract
The crude petroleum ether and chloroform extracts of Micromelum minutum (G. Frost.) Wright & Arn (Rutaceae) showed strong cytotoxic activity when tested against a T-lymphoblastic leukemia cell line. Further fractionation of the extracts resulted in the isolation of five new coumarins 3″,4″-dihydrocapnolactone, 2′,3′-epoxyisocapnolactone, 8-hydroxyisocapnolactone-29,39-diol, 8-hydroxy-3″,4″-dihydrocapnolactone-29,39-diol and 8,4″-dihydroxy-3″,4″-dihydrocapnolactone-29,39-diol, and two triterpenes. Some of these compounds were strongly active against T-lymphoblastic leukemia (CEM-SS), promyeolocytic leukemia (HL60), cervical cancer (HeLa) and liver cancer (HepG2) cell lines. 8-Hydroxyisocapnolactone-29,39-diol was found to be the most active with IC50 values of 2.9, 2.5, 6.9, and 5.9 μg/ml, respectively. This was followed by 2′,3′-epoxyisocapnolactone. When evaluated against the normal mouse fibroblast (3T3) cell line, 8-hydroxyisocapnolactone-29,39-diol was found to be inactive, hence it could serve as a valuable lead for further design and synthesis of more active analogues.
Introduction
Micromelum minutum (G. Rorst.) Wight & Arn (Rutaceae) is a small to medium-size shrub commonly found in the limestone forests of Peninsular Malaysia, Sabah, and Sarawak (CitationJones, 1995). According to Burkill (1935), the leaves are traditionally used to cure fever and giddiness, and the poultice of the boiled roots is applied to treat ague. In Indochina, the roasted and crushed leaves are rubbed on to skin irritated by scabies and are also considered to be emmenagogues (CitationPerry, 1980). The species is well known to contain coumarins, but depending on the collection location, the compounds isolated can also be different (CitationRahmani et al., 1994, Citation2003; CitationSusidarti et al., 2006; CitationTantivatana et al., 1983; CitationDas et al., 1984). The first sample collected in Peninsula Malaysia yielded micromelin and a new dihydrocinnamic acid derivative of micromelin, while another sample from a different location (also in Peninsular Malaysia) yielded tetracyclic microminutinin and 6-methoxymicrominutinin (CitationRahmani et al., 1994). However, the third sample collected in Sabah yielded five new capnolactone derivatives (CitationRahmani et al., 2003; CitationSusidarti et al., 2006). Coumarins have attracted intense interest in recent years because of their diverse pharmacological properties, and their cytotoxic properties have been extensively examined (CitationKostova, 2005; CitationBudzisz et al., 2003; CitationThornes et al., 1994). In this communication we wish to highlight the cytotoxic activity against cancer cell lines shown by four of the new capnolactone coumarins isolated from M. minutum collected in Sabah.
Materials and methods
General experimental procedures
The specimen of Micromelum minutum was collected in Sepilok, Sabah, Malaysia in 1999, and a sample specimen was deposited at the Forest Research Centre, Sepilok, Sabah (accession number SAN 142904 and authenticated by Julius Kulip of the Centre). Detailed extraction, isolation, and spectral data of compounds 1–7 have been reported in our previous publications (CitationRahmani et al., 2003; CitationSusidarti et al., 2006).
Cytotoxic assay
The crude extracts and isolated pure compounds from Micromelum minutum were screened for their cytotoxic activity by using microculture tetrazolium salt (MTT) assay (CitationMosmann, 1983). The cell lines used were CEM-SS (T-lymphoblastic leukemia), HL60 (human promyelocytic leukemia), HeLa (cervical cancer), HepG2 (liver cancer) and 3T3 (normal mouse fibroblast). CEM-SS was obtained from The National Cancer Institute, Frederick, MD, USA, while HL60, HeLa and 3T3 were obtained from RIKEN Cell Bank, Tsukuba, Japan. All cells were grown and maintained in RPMI 1640 (Sigma, USA) medium supplemented with 10% fetal bovine serum (Flow Lab, Australia), 100 IU/ml penicillin (Flow Lab) and 100 μg/ml streptomycin (Flow Lab) at 37°C, 5% CO2 and 90% humidity.
Cytotoxic assays were performed in 96-well flat bottom microwell plates (Nunc, Denmark). Various concentrations of the compounds were added to 96-well flat bottom microwell plates before the cells were seeded. One hundred μl of the compound at a concentration of 60 μg/ml were added into rows A and B, and a two-fold dilution gradient of the compounds was established through a series of mixing with the RPMI medium and aliquot transfers. Exponentially growing cell suspensions (100 μl), at a concentration of 5 × 105 cells/ml, were seeded into 96-well microplates in the presence of various concentrations of the compounds. Controls consisted of untreated cells only. The assays were performed in triplicate, and the culture plates were incubated for 3 days at 37°C in 5% CO2 humidified incubator.
After 3 days of incubation the fractions of surviving cells were determined relative to the untreated cell population by the colorimetric MTT (3-[4,5-dimethyl- thiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay. A volume of 20 μl MTT solution (5 mg/ml in PBS) was added to each well followed by 4 h incubation at 37°C. After incubation, 150 μl of the medium was removed from each well and formazan crystal formed was solubilized with 100 μl of dimethyl sulfoxide in each well. The plate was then left at room temperature for 15–30 min before reading the absorbance (optical density) of each well at 570 nm using an EL 340 microplate reader.
Assessment of the compounds’ cytotoxicity towards a normal mouse fibroblast (3T3) cell line was slightly different. The cells were first placed on 96-well flat bottom microwell plates, and drug treatment began only when the cells in each well were confluent. Treatment was performed by removing the medium from each well, and various concentrations of compounds were added as described above. The cells that were killed by a cytotoxic agent yielded less formazan production and lower optical density. A plot of the percentage of cell viability against concentration of the extract or compound gives a measure of cytotoxicity. The cytotoxic index used was IC50, which is the concentration that yields 50% inhibition of the cell viability compared with untreated control. Extracts or isolated compounds that exhibited cytotoxic index (IC50) < 10 μg/ml were considered to have significant cytotoxic activity.
Results and discussion
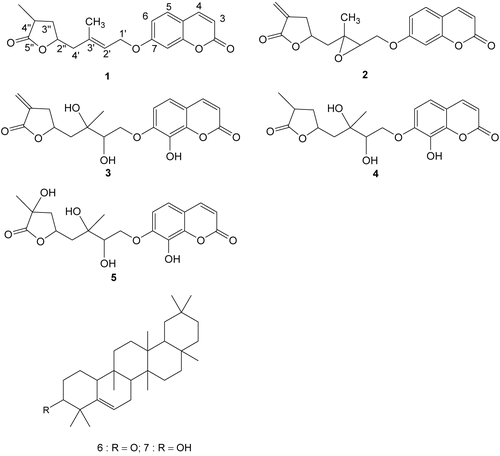
Both the leaf and bark extracts of M. minutum were assayed for cytotoxic activity against a T-lymphoblastic leukemia (CEM-SS) cell line. The chloroform extracts of the leaves showed strong activity, while the bark extracts had a moderate activity with IC50 values of 4.2 and 13.7 μg/ml, respectively. Further isolation of the chloroform extract of the leaves resulted in the identification of five coumarins 3″,4″-dihydrocapnolactone (1), 2′,3′-epoxyisocapnolactone (2), 8-hydroxyisocapnolactone-29,39-diol (3), 8-hydroxy-3″,4″-dihydrocapnolactone-29,39-diol (4) and 8,4″-dihydroxy-3″,4″-dihydrocapnolactone-29,39-diol (5) and two triterpenes 5(6)-gluten-3-one (6) and 5(6)-gluten-3α-ol (7). These have been reported previously (CitationRahmani et al., 2003; CitationSusidarti et al., 2006).
The cytotoxic activities of the crude extracts and the first four coumarins isolated from the chloroform extract of the leaves against four cell lines, as determined by microculture tetrazolium salt (MTT) assay (CitationMosmann, 1983), are shown in . Two of the compounds, 2′,3′-epoxyisocapnolactone (2) and 8-hydroxyisocapnolactone-29,39-diol (3) were significantly toxic to CEM-SS and HL60 cell lines. The IC50 value of the former against these two cancer cell lines were 3.9 and 4.2 μg/ml, respectively, while those of the latter were 2.9 and 2.5 μg/ml, respectively. Phase contrast photomicrographs of the T-lymphoblastic leukemia cell line taken from the 96-well plate one hour after addition of MTT showed early stage formation of formazan in viable cells. Upon addition of MTT, the cells became swollen and lost their original shapes before the formazan crystals were formed. However, the cytotoxicity of these compounds towards cervical cancer and liver cancer cell lines were found to be moderate, with IC50 values ranging from 5.0 to 10.0 μg/ml. Another coumarin, 3″,4″-dihydrocapnolactone (1), was only weakly toxic to the CEM-SS cell line, with an IC50 of 12.9 μg/ml, while the fourth compound, 8,4″-dihydroxy-3″,4″-dihydrocapnolactone-29,39-diol (5), was inactive. The high IC50 values obtained for compounds, (2) and (3), when tested against a normal mouse fibroblast (3T3) cell line, indicated that the normal cells were not sensitive to the compounds. These compounds could serve as valuable leads for further evaluation and synthesis of more potent agents.
Table 1. Cytotoxic activity of extracts, 3″,4″-dihydrocapnolactone (1), 29,39-epoxyisocapnolactone (2), 8-hydroxyisocapnolactone-29,39-diol (3) and 8,4″-dihydroxy-3″,4″-dihydrocapnolactone-29,39-diol (5).
Conclusion
Two of the five coumarins isolated from Micromelum minutum, 29,3′-epoxyisocapnolactone (2), 8-hydroxyisocapnolactone-29,39-diol (3), showed strong cytotoxic activity when tested against both T-lymphoblastic leukemia (CEM-SS) and promyelocytic leukemia (HL60) cell lines. The IC50 values of the former against these two cancer lines were 3.9 and 4.2 mg/ml, respectively, while those of the latter were 2.9 and 2.5 mg/ml, respectively. These compounds are both good candidates for further evaluation, in particular 8-hydroxyisocapnolactone-29,39-diol (3) as it does not show any effect on normal cells as demonstrated by assays against a normal mouse fibroblast (3T3) cell line.
Acknowledgements
We wish to thank the Malaysian Government for providing financial support under the IRPA programs and Universiti Putra Malaysia for providing the facilities.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Budzisz E, Brzezinska E, Krajewska U, Rozalski M (2003): Cytotoxic effects, alkylating properties and molecular modelling of coumarin derivatives and their phosphonic analogues. Eur J Med Chem 38: 597–603.
- Burkill IH (1966): A Dictionary of Economic Products of the Malay Peninsula, Vol. II. London, Crown Agents for the Colonies, p. 1493.
- Das S, Baruah RH, Sharma RP, Barua JN, Kulanthaivel P, Herz W (1984): 7-Methoxycoumarins from Micromelum minutum. Phytochemistry 23: 2317–2321.
- Jones DT (1995). Rutaceae. In: Soepadmo E, Wong KM, eds., The flora of Sabah and Sarawak. Kuala Lumpur, Ampang Press Sdn Bhd, pp. 351–401.
- Kostova I (2005): Synthetic and natural coumarins as cytotoxic agents. Cur Med Chem – Anti-cancer Agents 5: 29–46.
- Mosmann T (1983): Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assay. J Immunol Methods 65: 55–58.
- Perry LM, Metzger J (1980): Medicinal Plants of East and Southeast Asia. Cambridge, MA, MIT Press, pp. 366–367.
- Rahmani M, Susidarti RA, Ismail HBM, Sukari MA, Taufiq-Yap YH, Ee GCL, Ali AM, Kulip J, Waterman PG (2003): Coumarins from Malaysian Micromelum minutum. Phytochemistry 64: 873–877.
- Rahmani M, Taufiq-Yap YH, Ismail HBM, Sukari MA, Waterman PG (1994): New coumarin and dihydrocinnamic acid derivatives from two Malaysian populations of Micromelum minutum. Phytochemistry 37: 561–564.
- Susidarti RA, Rahmani M, Ismail HBM, Sukari MA, Taufiq-Yap YH, Ee GCL, Ali AM, Kulip J, Waterman PG (2006): A new coumarin and triterpenes from Malaysian Micromelum minutum. Nat Prod Res 20: 145–151.
- Tantivatana P, Ruangrungsi N, Vaisiriroj V, Lankin DC, Bhacca NS, Borris RP, Cordell GA Johnson LF (1983): Microminutin, a novel cytotoxic coumarin from Micromelum minutum (Rutaceae). J Org Chem 48: 268–270.
- Thornes RD, Daly L, Lynch G, Breslin B, Browne H, Browne HY, Corrigan T, Daly P, Edwards G, Gaffney E (1994): Treatment with coumarin to prevent or delay recurrence of malignant melanoma. J Cancer Res Clin Oncol 120: S32–S34.