Abstract
In our continuous study of the Malvaceae family, we describe here the isolation of four triterpenes (frideline, lupeol, cycloartenol, and cycloeucalenol), a steroid (β-sitosterol), and four phenolic compounds from the aerial parts of Herissantia tiubae (K. Schum.) Brizicky (i.e., a benzoic acid derivative, a coumarin and two flavonoids, kaempferol 7-O-α-l-rhamnopyranoside and 4′,5-dihydroxy-3,6,7,8,3′-pentamethoxyflavone). The structural identification of the compounds was made by comparison with literature data and spectral analysis, including two-dimensional NMR techniques. These compounds are reported here for the first time in genus Herissantia. The pentamethoxyflavone was assayed against strains of Staphylococcus aureus possessing efflux mechanisms of resistance to norfloxacin, tetracycline, and erythromycin. Although the compound did not display relevant antibacterial activity (MIC ≥ 512 μg/mL), it modulated the activity of antibiotics, i.e., in combination with antibiotics (at 128 μg/mL), a two-fold reduction in the MIC values for tetracycline and erythromycin, a 32-fold reduction in the MIC for norfloxacin were observed.
Keywords::
Introduction
According to the new phylogenetic circumscription, the Malvaceae family consists of about 243 genera and 4225 species (CitationStevens, 2003), which are widespread in many regions of the world, particularly in tropical areas, mainly in South America (CitationHeywood, 1993). Species of Malvaceae are used in folk medicine for many applications, such as a diuretic, in the treatment of rheumatism, gastrointestinal disorders (CitationSchultz, 1968; CitationYesilada & Gürbüz, 2002), snakebites (CitationOtero et al., 2000) and asthma (CitationFranzotti et al., 2000). Anti-inflammatory and antinociceptive effects were also investigated (CitationVenkatesh et al., 1999). Phytochemical investigations of Malvaceae species have described the isolation of fatty acids (CitationCarmody et al., 1945; CitationVickery, 1980; CitationSchimid & Patterson, 1988; CitationNakatani et al., 1986), essential oils (CitationAmes & Macleod, 1990), sesquiterpenelactones (CitationSharma & Ahmad, 1989), triterpenes (CitationAhmed et al., 1990) and flavonoids (Silva et al., 2005a, 2005b), among many other compounds.
The genus Herissantia comprises six species, restricted to the tropical America with the major occurrence in Mexico, Antilles, and South America. Only Herissantia crispa L. (Brizicky) is widespread, occurring from the United States to Argentina. Herissantia tiubae (K. Schum) Brizicky, commonly known as mela bode, consists of a small shrub, found in the Brazilian northeast, mainly between the states of Bahia and Pernambuco (Corrêa, 1978). In previous publications concerning Herissantia tiubae, we described the isolation and structural identification of four polyoxygenated flavonoids (Silva et al., 2005a), to which has been attributed a broad spectrum of biological activity (CitationChen et al., 1997). In a different study we isolated two flavonol glycosides, kaempferol 3,7-di-O-α-l-rhamnopyranoside and kaempferol 3-O-β-D-(6′′-E-p-coumaroyl) glucoside (Silva et al., 2005b). Kaempferol 3,7-di-O-α-l-rhamnopyranoside was subjected to preliminary pharmacological tests, showing a relaxing effect over superior mesenteric artery (Silva et al., 2005b), which suggested a possible cardiovascular effect. Cycloeucalenol and cycloartenol, previously isolated from H. tiubae, showed a relaxant effect in the guinea-pig ileum (CitationGomes et al., 2005).
Efflux pumps are integral proteins of bacterial membrane accounting for much of bacterial resistance since they extrude antibiotics from the cell (CitationPiddock, 2006). Resistance modifying agents are compounds that potentiate the activity of an antibiotic against resistance strains, and some of these agents may act as an inhibitor of efflux pumps (EPIs). Plants provide a rich source of EPIs and several compounds have been identified as potent inhibitors (CitationGibbons, 2004). In this work, we evaluated 4′,5-dihydroxy-3,6,7,8,3′-pentamethoxyflavone as modulator of antibiotic resistance using strains of Staphylococcus aureus possessing efflux pumps.
Materials and methods
Equipment
NMR spectra (1H, 13C, HMQC, HMBC, COSY) were run on a Mercury-Varian instrument operating at 200 MHz and 50 MHz for 1H and 13C, respectively, and also on a Brucker-AC operating at 300 (1H) and 75 MHz (13C). The NMR data were measured in CDCl3, CD3OD and pyridine-d6, and chemical shifts were expressed in parts per million (ppm) with reference to the solvent signal. Chromatography columns were used with silica gel (Merck) and Sephadex LH-20 (Pharmacia, Uppsala, Sweden). TLC was preformed with silica gel 60 F254 (Merck); spots were visualized under UV light (244 and 366 nm), exposure to iodine vapor and by spraying with 5% FeCl3 reagent (for detection of glycosyl flavonoids).
Plant material
The aerial parts were collected in CitationJanuary 2004 in the county of Juazeirinho, Paraíba state, Brazil. It was identified by Maria de Fátima Agra of the Universidade Federal da Paraíba. A voucher specimen (no. 2434) is deposited in the Herbarium Lauro Pires Xavier-JPB at the same university.
Extraction and isolation
Dried and ground aerial parts of H. tiubae (5000 g) were extracted with ethanol (3 X 10 L, 72 h each) and the crude extract was concentrated to dryness under reduced pressure to yield 190 g of a dark green residue. This material was partitioned with hexane, chloroform, ethyl acetate, and butanol. The hexane extract (20 g) was chromatographed on silica gel and eluted with hexane, gradually increasing the polarity with CHCl3 and then MeOH, yielding 10 major fractions after analysis by TLC (SiO2, hexane with increasing CHCl3, iodine vapor). Fraction 3 was also subjected to chromatography over silica gel, using hexane, CHCl3 and MeOH, resulting in 88 fractions, also combined by TLC analysis. Subfractions 05-13 and 14-57 led to isolation of compounds 1 and 2. Fraction 6, after two sucessive chromatographies on silica gel, yielded compounds 3, 4, and 5.
The CHCl3 phase (27 g) was chromatographed over silica gel 60 and eluted with hexane, CHCl3 and MeOH with increasing polarity, resulting in 100 fractions, which were combined after analysis by TLC (SiO2, hexane:CHCl3 9:1, iodine vapor). Fractions 50-55 were subjected to repeated chromatographic procedures on silica gel 60, using hexane, CHCl3 and MeOH, yielding compounds 6, 7, and 8, which were purified after recrystallization with hexane:CHCl3 (9:1). Ethyl acetate extract (1.5 g) was applied to a Sephadex LH-20 column (Pharmacia, Uppsala-Sweden) with MeOH as eluent, where fractions of 20 mL were obtained and combined after analysis through TLC (SiO2, CHCl3:MeOH 1:1, FeCl3 5%). Fractions 13/16 were recrystallized with MeOH furnishing compound 9.
Bacterial strains
The strains of S. aureus used were SA-1199B, which overexpresses the norA gene encoding the NorA fluoroquinolones (and other drugs) efflux protein (CitationKaatz et al., 1993; CitationKaatz & Seo, 1995), RN4220 harboring plasmid pUL5054, which carries the gene encoding the MsrA macrolide efflux protein (CitationRoss et al., 1989), and IS-58, which possesses the TetK tetracycline efflux protein (CitationGibbons & Udo, 2000). All strains were maintained on blood agar base (Difco) slants and, prior to use (assay), the cells were grown overnight at 37°C in brain heart infusion broth (BHI, Difco).
Antibiotics and 4′,5-dihydroxy-3,6,7,8,3′-pentamethoxyflavone
Norfloxacin, erythromycin and tetracycline were obtained from their respective manufacturers. The stock solutions of the antibiotics were prepared according to CitationCLSI guidelines (2005). The stock solution of 4′,5-dihydroxy-3,6,7,8,3′-pentamethoxyflavone was prepared in DMSO, and its highest concentration remaining after dilution into broth (4%) caused no inhibition of bacterial growth.
Drug susceptibility testing
The minimum inhibitory concentrations (MICs) of the antibiotics and 4′,5-dihydroxy-3,6,7,8,3′-pentamethoxyflavone were determined in BHI by the microdilution assay using a suspension of ca. 105 cfu/mL and a drug concentration range of 256 to 0.5 μg/mL (two-fold serial dilutions). The MIC is defined as the lowest concentration at which no growth is observed. For the evaluation of 4′,5-dihydroxy-3,6,7,8,3′-pentamethoxyflavone as a modulator of antibiotic resistance, the MICs of the antibiotics were determined in the presence of the compound at a sub-inhibitory concentration.
Results and discussion
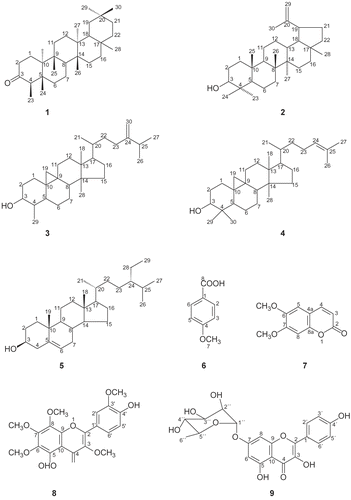
The structure identification of compounds 1-7 was based on spectral analysis and also by comparison with literature data, which allowed their assignments as friedeline (1) (CitationCheung & Willianson, 1969), lupeol (2) (CitationVanderlei, 1985), cycloeucalenol (3) (CitationAkihisa et al., 1989), cycloartenol (4) (CitationEmmons et al., 1989), β-sitosterol (5) (CitationKojima et al., 1990), p-methoxybenzoic acid (6) (CitationMarques et al., 1998) and 6,7-dimethoxycoumarin (7) (CitationInuma et al., 1993).
The IR spectrum of compound 8 showed a hydroxyl signal at 3368 cm−1, a conjugated carbonyl ester absorbance at 1647 cm−1 and aromatic C = C stretching absorptions at 1593 and 1466 cm−1. The 1H NMR spectrum showed characteristic signals of a flavonoid compound. An ABX pattern in the B-ring was revealed by the three protons resonances at δ 7.74 (1H, dd, J = 8.2 and 1.7 Hz), δ 7.76 (1H, J = 1.7 Hz) and δ 7.02 (1H, d, J = 8.2 Hz), which could be assigned to H-6´, H-2´ and H-5´, respectively, as found in 3′,4′-oxygenated flavonoids (CitationChen et al., 1997). Five methoxyl signals were observed at δ 3.84-4.07, suggesting that compound 8 was a polymethoxyflavone. The proton signal at δ 6.20 was suggestive of a phenolic hydroxyl at C-4′ and the low-field singlet at δ 12.36 is characteristic of the chelated 5-OH.
The 13C NMR spectrum of 8 showed four aromatic methoxyls, with considerable downfield chemical shifts at δ 60.1-62.0, that suggest these groups are attached to di-ortho-substituted carbons (CitationRoitman & James, 1985) and possibly located on the A-ring and C-ring. The resonance at δ 56.0 could be assigned to the methoxyl group located at C-3′, considering this chemical shift is characteristic of an aromatic methoxyl attached to carbon bearing one or no ortho substituent.
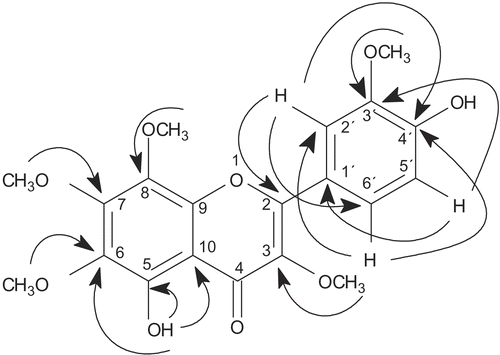
The unambiguous assignments of carbons and hydrogens of compound 8 was possible based on the analysis of the HMBC cross correlations. The attribution of chemical shifts in rings A and C were made according to the following correlations: OH-5 with C-5 (δ 149.1) (2J), C-6 (δ 136.2) (3J) and C-10 (107.4) (3J); CH3O-6 (δ 3.92) with C-6 (δ 136.2) (3J), CH3O-7 (δ 4.07) with C-7 (δ 152.9) (3J), CH3O-8 (δ 3.93) with C-8 (δ 132.8) (3J) and CH3O-3 (δ 3.84) with C-3 (δ 138.6) (3J). Ring B was assigned by the interactions between H-2′ (δ 7.76) with C-2 (δ 155.9) (3J), C-4′ (δ 148.7) (3J) and C-6′ (δ 122.9) (3J); H-5′ (δ 7.02) with C-1′ (δ 122.5) (3J) and C-3′ (δ 146.5) (3J) and H-6′ (δ 7.74) with C-2 (δ 155.9) (3J), C-4′ (δ 148.7) (3J) and C-2′ (δ 110.9) (3J). All the correlations are shown in . On the basis of the above evidence, compound 8 was identified as 5,4′-dihydroxy,3,6,7,8-3′-pentamethoxyflavone. Its chemical shifts were also compared with literature data (CitationSharaf et al., 1992).
Table 1 . 1H and 13C NMR chemical shifts assignments for compound 8.
The 1H NMR spectrum of compound 9 showed a group of signals for kaempferol and a sugar unit. Kaempferol was indicated by two doublets at δ 6.38 and δ 6.70 (J = 2.2 Hz) for H-6 and H-8 respectively and a pair of doublets at δ 8.06 and δ 6.88 (J = 8.9 Hz) for H-2′,6′ and H-3′,5′, respectively, referring to an AA′,BB′ system. The sugar region of the 1H NMR spectrum revealed a doublet at δ 5.24 (J = 1.6 Hz) related to the anomeric proton H-1′′ of rhamnose. The other sugar protons resonated at δ 4.02 (dd, J = 3.4, 1.6 Hz, H-2′′), 3.83 (dd, J = 9.2, 3.4 Hz, H-3′′), 3.43 (t, J = 9.4 Hz, H-4′′) and their unambiguous assignments were further confirmed with the help of COSY and NOESY experiments. The HMBC spectrum proved that the rhamnosyl unit was attached at C-7 due to the correlation between the anomeric proton at δ 5.24 and the carbon at δ 163.2 (C-7) (3J). Supporting this information were the interactions between H-6 (δ 6.38) and H-8 (δ 6.70) with C-7 (2J). Thus, the structure of 9 was assigned as kaempferol 7-O-α-l-rhamnopyranoside (CitationKaouadji, 1990).
4′,5-Dihydroxy-3,6,7,8,3′-pentamethoxyflavone showed no antibacterial activity at 256 μg/mL against any strains of S. aureus used (MIC ≥ 512 μg/mL). When the compound was incorporated into the growing medium at 128 μg/mL (≤ ¼ MIC), a two-fold reduction in the MIC was observed for tetracycline and for erythromycin and a dramatic reduction of the MIC for norfloxacin ().
Table 2. MIC values (μg/mL) of test strains for antibiotics in the absence and (presence) of 4′,5-dihydroxy-3,6,7,8,3′-pentamethoxyflavone (128 μg/mL).
4′,5-Dihydroxy-3,6,7,8,3′-pentamethoxyflavone modulated the activities of the antibiotics by reducing the concentration of antibiotic needed to inhibit the growth of the drug resistant (effluxing) bacteria. This activity may be related to its lipophilicity, a common feature of several putative efflux pump inhibitors. This quality, as pointed out by CitationGibbons (2004), is likely to be important for its solubility in the bacterial membrane and binding to the efflux transporters before inhibition can occur.
Some methoxylated flavones that potentiate the activity of antimicrobial drugs have been described (CitationStermitz et al., 2002). However, as far as we know, neither a pentamethoxyflavone nor a methoxylated flavone from the Malvaceae had been previously evaluated. Thus, the results here presented indicate that H. tiubae (and broadly Malvaceae) could serve as a source of plant-derived natural products that modulate bacterial multidrug resistance, i.e., a source of potential adjuvant of antibiotics.
Compound 8 (4′,5-dihydroxy-3,6,7,8,3′-pentamethoxyflavone): Yellow needles (hexane-CHCl3), IR νKBrmax cm−1: 3368, 3004, 2940, 2836, 1282, 1050. 1H NMR (CDCl3): see . 13C NMR (CDCl3): see . NMR data agree with literature (CitationSharaf et al., 1992).
Compound 9 (kaempferol 7-O-α-l-rhamnopyranoside): Yellow powder (MeOH). 1H NMR (CDCl3): δ 8.06 (2H, d, J = 8.9 Hz, H-2´/6´), 6.88 (2H, d, J = 8,9 Hz, H-3´/5´), 6.70 (1H, d, J = 2.2 Hz, H-8), 6.38 (1H, d, J = 2.2 Hz, H-6), 5.54 (1H, d, J = 1.6 Hz, H-1′′), 4.03 (1H, dd, J = 3.4, 2 Hz, H-2′′), 3.83 (dd, J = 9.2, 3.4 Hz, H-3′′), 3.60 (1H, dd, J = 9.4, 6 Hz, H-5′′), 3.43 (1H, t, J = 9.4 Hz, H-4′′), 1.25 (1H, d, J = 5.8 Hz, H-6′′). 13C NMR (CDCl3): δ 177.4 (C-4), 163.2 (C-7), 162.3 (C-28), 160.7 (C-4´), 157.3 (C-9), 148.7 (C-2), 137.5 (C-3), 130.8 (C-2´/6´), 123.5 (C-1´), 116.3 (C-3´/5´), 106.1 (C-10), 99.9 (C-6), 99.8 (C-1´´), 95.32 (C-8), 73.6 (C-4′′), 72.0 (C-3′′), 71.7 (C-2′′), 71.2 (C-5´´), 18.0 (C-6´´). NMR chemical shifts agree with literature (CitationKaouadji, 1990).
Acknowledgements
J.P.S-J. and V.S.F-S. are very grateful to Dr. Simon Gibbons (University of London) for his valuable and kind cooperation. CNPq (PIBIC/UFPb) is thanked for the award of an undergraduate studentship. This work was supported by the following Brazilian agencies: CNPq and CAPES.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahmed ZK, Kazmi SN, Malik AA (1990): A new pentacyclic triterpene from Abutilon pakistanicum. J Nat Prod 33: 1342–1344.
- Akihisa T, Yokota T, Takahashi N, Tamura T, Matsumoto T (1989): 25-Methylgramisterol and other 4α-mtehylsterols for Phaseolus vulgaris seeds. Phytochemistry 28: 1219–1224.
- Ames JM, Macleod G (1990): Volatile components of okra. Phytochemistry 29: 1201–1207.
- Carmody DR, Dejong W, Smith TR (1945): Buttonweed seed oil: A source of linoleic acid. Oil & Soap 22: 263–265.
- Chen J, Montanari AM, Widmer WW (1997): Two new polymethoxylated flavones, a class of compounds with potential anticancer activity, isolated from cold pressed dancy tangerine peel oil solids. J Agr Food Chem 45: 364–368.
- Cheung TJ, Willianson DG (1969): NMR signals of methyl groups of triterpenes with oxygen functions at position 2,3 and 23. Tethrahedron 25: 229–259.
- CLSI – Clinical and Laboratory Standards Institute/NCCLS (2005): Performance Standards for Antimicrobial Susceptibility Testing; Fifteenth Informational Supplement. CLSI/NCCLS document M100-S15. Wayne, PA, Clinical and Laboratory Standards Institute.
- Corrêa P (1978): Dicionário de Plantas úteis do Brasil e das Exóticas Cultivadas, fifth editon. Rio de Janeiro, Imprensa Nacional, pp. 70–71.
- Emmons GT, Wilson WK Jr, Schroepfer GJ (1989): 1H and 13C assignments for lanostan-3β-ol derivatives: Revised assignments for lanosterol. Magn Reson Chem 27:1 012–1024.
- Franzotti EM, Santos CVF, Rodrigues HMSL, Mourão RHV, Andrade MR, Antoniolli AR (2000): Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L. (Malva-branca). J Ethnopharmacol 72: 273–278.
- Gibbons S (2004): Anti-staphylococcal plant natural products. Nat Prod Rep 21: 263–277.
- Gibbons S, Udo EE (2000): The effect of reserpine, a modulator of multidrug efflux, on the in vitro activity of tetracycline against clinical isolates of methicillin resistance Staphylococcus aureus (MRSA) possessing the tet (K) determinant. Phytother Res 14: 139–140.
- Gomes AYS, Souza MFV, Cortes SF, Lemos VS (2005): Mechanism involved in spasmolytic effect of a mixture of two triterpenes, cycloartenol and cycloeucalenol, isolated from Herissantia tiubae in the guinea-pig ileum. Planta Med 71: 1025–1029.
- Heywood VH (1993): Flowering Plants of World, second edition. London, Batsford, pp. 59–60.
- Inuma M, Ohyama M, Tanaka T, Mizuno M, Hong SK (1993): Five flavonoid compounds from Echinosophora koreensis. Phytochemistry 33: 1241–1245.
- Kaatz GW, Seo SM, Ruble CA (1993): Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother 37: 1086–1094.
- Kaatz GW, Seo SM (1995): Inducible NorA-mediated multidrug resistance in Staphylococcus aureus. Antimicrob Agents Chemother 39: 2650–2655.
- Kaouadji M (1990): Acylated and non-acylated kaempferol monoglycosides from Platanus acerifolia buds. Phytochemistry 29: 2295–2297.
- Kojima H, Sato N, Hatano A, Ogura H (1990): Sterol glucosides from Prunella vulgaris. Phytochemistry 29: 2351–2355.
- Marques DD, Machado MIL, Carvalho MG, Meleira LAC, Braz-Filho R (1998): Isoflavonoids and triterpenoids isolated from Pterodon polygalaeflorus. J Braz Chem Soc 9: 295–301.
- Nakatani M, Fukunaga Y, Hase T (1986): Two aliphatic enone ethers from Hibiscus rosa-sinensis. Phytochemistry 25: 449–452.
- Otero R, Núñez V, Barona J, Fonnegra R, Jiménez SL, Osorio RG (2000): Snakebites and ethnobotany in the northwest region of Colombia. Part III: Neutralization of the haemorrhagic effect of Bothrops atrox venom. J Ethnopharmacol 73: 233–241.
- Piddock LJV (2006): Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev 19: 382–402.
- Ross JI, Farrell AM, Eady EA, Cove JH, Cunliffe WJ (1989): Characterisation and molecular cloning of the novel macrolide-streptogramin B resistance determinant from Staphylococcus epidermidis. J Antimicrob Chemother 24: 851–862.
- Roitman JN, James LF (1985): Chemistry of toxic range plants. Higly oxygenated flavonol methyl ethers from Gutierezia microcephala. Phytochemistry 24: 835–848.
- Schimid KM, Patterson GW (1988): Distribution of cyclopropenoid fatty acids in Malvaceous plant parts. Phytochemistry 27: 2831–2834.
- Schultz AR (1968): Introdução ao Estudo da Botânica Sistemática, third edition. Porto Alegre, Globo, pp. 60–64.
- Sharaf M, Mansour RMA, Saleh NAM (1992): Exudate flavonoids from aerial parts of four Cleome species. Biochem Sys Ecol 20: 443–448.
- Sharma PV, Ahmad ZA (1989): Two sesquiterpene lactones from Abutilon indicum. Phytochemistry 28: 3525–3525.
- Silva DA, Chaves COM, Costa DA, Moraes MRR, Nóbrega FBP, Souza MFV ((2005)a): Flavonoids from Herissantia tiubae. Pharm Biol 43: 197–200.
- Silva DA, Costa DA, Silva DF, Souza MFV, Agra MF, Medeiros IA, Barbosa-Filho JM, Braz-Filho R ((2005)b): Glycosyl flavonoids from Herissantia tiubae (K. Schum) Brizicky (Malvaceae) and preliminary tests of kaempferol 3,7-di-O-α- l-rhamnopyranoside. Brazilian J Pharmacog 15: 23–29.
- Stevens PF (2003): Angiosperm phylogeny website. Version 4, available at http://www.mobot.mobot.org, accessed May (2008).
- Stermitz FR, Scriven LN, Tegos G, Lewis K (2002): Two flavonols from Artemisa annua which potentiate the activity of berberine and against a norfloxacinresistant strain of Staphylococcus aureus. Planta Med 68: 1140–1141.
- Vanderlei MF (1985): Estudo químico e farmacológico de Himatanthus phagedaenica (Mart.) Woodson, João Pessoa, Dissertação de Mestrado (Química e Farmacologia de Produtos Naturais), Universidade Federal da Paraíba, pp. 91–95.
- Venkatesh S, Reddy SR, Suresh B, Ressy BM, Ramesh M (1999): Antinociceptive and anti-inflammatory activity of Sida rhomboidea leaves. J Ethnopharmacol 67: 229–232.
- Vickery JR (1980): The fatty acid composition of seed oils from ten plant families with particular reference to cyclopreopene and dihydrosterculic acids JAOCS 57: 87–91.
- Yesilada E, Gürbüz IA (2002): Compilations of the Studies on the Anti-Ulcerogenic Effects of Medicinal Plants. Recent Progress in Medicinal Plants. Houston, SCI Tech Publishing LLC, pp. 111–173.