Abstract
Diabetes mellitus is a chronic disease which affects millions of people worldwide. The prevalence of this disease is increasing annually and the number of diabetics is projected to rise above 300 million before 2025. The growing number of diabetics, coupled with the harsh side effects of some synthetic drugs has led to the increasing search for more natural products of plant origin. Vernonia amygdalina Del. (Asteraceae) is one of the plants commonly used for the treatment of diabetes in Africa. This study evaluated the effect of leaf extracts of this plant on glucose utilization in 3T3-L1, C2C12 muscle, and Chang-liver cells. Treatment of the cells with the acetone, methanol, water, and n-hexane/isopropanol extracts of V. amygdalina leaves significantly increased glucose utilization in the C2C12 muscle and Chang-liver cells but showed no effect on the 3T3-L1 cells. The water and n-hexane/isopropanol extracts were the most active in the C2C12 cells with a response of 78.3 and 95.6% above the control, respectively, while in the Chang-liver cells, water and acetone extracts had a response of 65.8 and 59.6% above the control, respectively. The results, especially of the water extract, strongly corroborate the ethnomedical uses of V. amygdalina as an antidiabetic plant.
Introduction
Diabetes mellitus is a common disease caused by the abnormality of carbohydrate metabolism which is linked to low insulin level or insensitivity of target organs to insulin. The disease currently affects about 160 million people worldwide, and the number is projected to rise above 300 million before 2025 (CitationBailey & Day, 2004; CitationLi et al. 2004; CitationMaiti et al. 2004). There are two types of diabetes, coded type 1 and type 2. The latter is the most prevalent type of the disease and is alleged to have reached epidemic proportions (CitationBailey & Day, 2004). Insulin resistance is implicated in type 2 diabetic patients. Its symptoms include decreased stimulation of muscle glycogen synthesis, defects in glycogen synthase activity, hexokinase activity, and decreased glucose uptake (CitationCline et al. 1999). One of the main disturbances of nutrient metabolism in type 2 diabetes is the impairment of the insulin-stimulated glucose utilization, especially by skeletal muscle cells (CitationBailey & Day, 2004).
Medicinal plants with the ability to stimulate glucose utilization in the body may be beneficial in developing long term and health friendly treatment for type 2 diabetes mellitus. This is due to the fact that many synthetic antidiabetic drugs have shown some side effects such as lactic acid intoxication and gastrointestinal upsets (CitationLi et al. 2004). Several plants have been reported to be used for the treatment of diabetes (CitationMarles & Farnsworth, 1995; CitationErasto et al. 2005). An example of such plants is Vernonia amygdalina Del. This plant is prominently used in Nigeria, Ghana, and South Africa for the treatment of diabetes (CitationAkah & Ekekwe, 1995; CitationErasto et al. 2005). It is further used for treatment of various infectious diseases such as venereal diseases, wounds, hepatitis, amoebic dysentery, and gastrointestinal problems (CitationHuffman et al. 1996; CitationErasto et al. 2006). The in vivo antidiabetic assay of the aqueous extracts from the leaves of this plant has indicated some blood sugar lowering effect in rats (CitationUhegbu, 2004). However, the mode of action of the extracts in lowering blood sugar in diabetic rats was uncertain. Based on this, the present study was designed to investigate the ability of the extracts from the leaves of V. amygdalina to stimulate glucose utilization in the 3T3-L1, C2C12 muscle, and Chang-liver cells. The three cell lines were used as prototypes for fat, muscle and liver cells. These are the representatives of the three major target tissues in the treatment of insulin resistance.
Materials and Methods
Cell lines, media and chemicals
3T3-L1, C2C12 and Chang-liver cell lines were obtained from the American Type Culture Collection (ATCC). Glucose oxidase reagent (SERA-PAK Plus, Bayer), and RPMI 1640 medium was supplied by Sigma Aldrich, S. Africa, while fetal calf serum (fcs) was purchased from Highveld Biological, S. Africa. The other chemicals were purchased from Merck Chemicals (Pty) Ltd, S. Africa.
Collection, extraction and preparation of plant materials
The leaves of V. amygdalina were harvested in March 2006 from a cultivated population in East London, South Africa. The authentication of the plant was done by Prof. Afolayan and a voucher specimen (Erasto 12) deposited in the herbarium of the University of Fort Hare. Leaves were allowed to dry under room temperature before pulverization using a blender. The ground materials were divided into four portions. A portion was extracted with n-hexane/isopropanol (3:1) while the rest were separately shaken in acetone, methanol, and water for 24 h. Later, the three extracts were concentrated to dryness under reduced pressure, while the water extract was freeze dried. Dried extracts were reconstituted and sterilized in diluted DMSO, vortexed and left for at least 15 min before further dilution with the respective growth medium for chronic exposure, or incubation medium for acute exposure (see glucose utilization assays). The final DMSO concentration never exceeded 0.25%.
Cell culture and glucose utilization assays
The method described by Van de Venter et al. (Citation2006) was used to determine the glucose utilization in 3T3-L1, C2C12 and Chang-liver cell lines. All incubations for maintenance of cells and glucose utilization were performed at 37°C in a humidified incubator with 5% CO2.
3T3-L1 cells
3T3-L1 cells were cultured in DMEM (1.5 g/L NaHCO3) with 10% fcs. For glucose utilization determination, 3T3-L1 preadipocytes were seeded at a density of 3000 cells per well into a 96-well plate and cultured for five days in a growth medium. After seeding, cells were not fed until the day of the glucose utilization experiment to allow the depletion of glucose from the medium. On day three after seeding, 1 μl of the extract was added to the appropriate wells to give a final extract concentration of 12.5 μg/ml. On day five after seeding, the medium was removed and 50 μl of incubation medium (8 mM glucose RPMI 1640 + 0.1% BSA) containing the V. amygdalina extract (50 μg/ml) was added to the appropriate wells, while the control wells contained incubation medium only. As the positive control, 1 μM of insulin was used. After 90 min of incubation, 10 μl was removed and placed into a new 96-well plate into which 200 μl glucose oxidase reagent (SERA-PAK Plus, Bayer) was added. After incubation for 15 min at 37°C, the absorbance was measured at 492 nm using a Multiscan MS microtiter plate reader (Labsystems). To calculate glucose utilization, the amount of glucose left in the medium after incubation was subtracted from the amount given at time 0 (8 mM). Cell viability in the representative wells was assessed using MTT (CitationMosmann, 1983) to determine cytotoxicity and to normalise glucose utilization results.
Chang-liver cells
Chang-liver cell lines were maintained in antibiotic-free growth medium consisting of RPMI 1640 (Sigma, S. Africa) supplemented with 10% fetal calf serum (Highveld Biological, South Africa). For the determination of glucose utilization, the liver cells were seeded into 96-well plates at a density of 6000 cells per well, and cultured for five days in a growth medium. Further treatment of cells was as described in 3T3-L1 cells, except that the incubation period for glucose utilization experiment was three hours for Chang-liver cells as opposed to 90 min for 3T3-L1 cells and the positive control for Chang-liver cells was 1 μM of Metformin. The glucose utilization was also calculated as in 3T3-L1 assay above.
C2C12 muscle cells
C2C12 muscle cells were maintained in antibiotic-free growth medium consisting of RPMI 1640 (Sigma, S. Africa) supplemented with 10% fetal calf serum (Highveld Biological, South Africa). For determination of glucose utilization, the muscle cells were seeded into 96-well plates at a density of 5000 cells per well, and cultured for four days in a growth medium. C2C12 cells were not exposed to extracts prior to the glucose utilization experiment. On day four after seeding, the glucose utilization experiment was performed as described 3T3-L1 cells, with the extract concentration of 50 μg/ml and an incubation time of 1 h. Insulin (1 μM) was used as the positive control. Cell viability was not determined because the cells were only exposed to the extracts for 1 h and, therefore, cell numbers would not have been affected.
Data analysis
All the results were expressed as percentages of the control value. The number of replicates per experiment is indicated in the figure captions. Data points are the mean ± SD. All glucose utilization and toxicity results were compared with the relevant control using the Student’s t-test and a p value less than 0.05 was considered significant.
Results
3T3-L1 cells
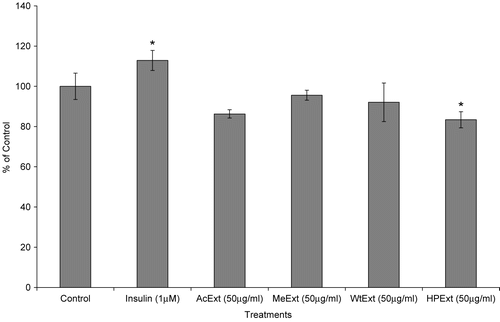
In this study, all extracts showed weak or no effect on glucose utilization in the fat cells (). Again, the MTT assay indicated that the 48 h of 3T3-L1 cells exposure to 50 μg/ml of n-hexane/isopropanol extract was toxic with a relative cell number of 83.4% compared to 100% for the control (p < 0.05) (). Glucose utilization in 3T3-L1 cells is regarded as one of the best in vitro models that represent an important mode of action for glucose utilization and disposal in mammals (CitationAnaga et al. 2004). Plant extracts which stimulate glucose metabolism in the cells are considered to be active. Therefore, these results indicate that the extracts have no effect in increasing glucose metabolism in the fat cells.
Figure 1. The effect of V. amygdalina extracts on glucose utilization in the 3T3-L1 cells. Cells were exposed to 12.5 μg/ml extract for 48 h prior to and 50 μg/ml during the glucose utilization experiment. Data represents the mean ± SD (n = 6). AcExt: acetone extract; MeExt: methanol extract; WtExt: water extract; HPExt: n-hexane/isopropanol extract. **p < 0.01 compared to control.
C2C12 muscle cells
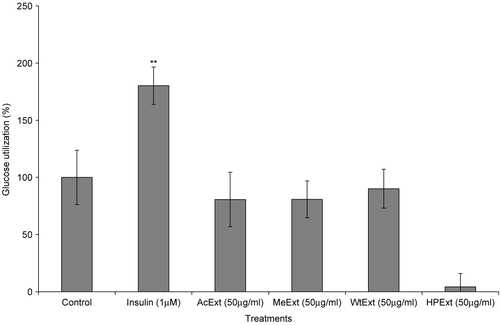
The use of C2C12 cell lines in this experiment was based on the fact that muscle cells are important targets of insulin action in terms of post-prandial glucose disposal. Also, the insulin resistance in the skeletal muscle has been one of the major problems of type 2 diabetes mellitus (CitationSchmitz-Peiffer et al. 1999). According to van de Venter et al. (2006), extracts with the ability to increase glucose utilization in the muscle cells by 150% and above, are considered to be very active. In this experiment, methanol, water and n-hexane/isopropanol extracts exhibited high activity with the response of 159.8, 178.3 and 195.6% of glucose utilization, respectively (). The acetone extract was the least active with a response of 145.2%. Consequently, the activity order in this assay was: acetone<methanol< water<hexane/isopropanol extract. These results suggest that the leaf extract of V. amygdalina have the ability to fight insulin resistance in the skeletal muscle tissues.
Figure 3. The effect of V. amygdalina extracts on glucose utilization in the C2C12 muscle cells. Cells were exposed to 50 μg/ml during the glucose utilization experiment. Data represents the mean ± SD (n = 10). AcExt: acetone extract; MeExt: methanol extract; WtExt: water extract; HPExt: n-hexane/isopropanol extract. *p < 0.05 compared to control.
Chang-liver cells
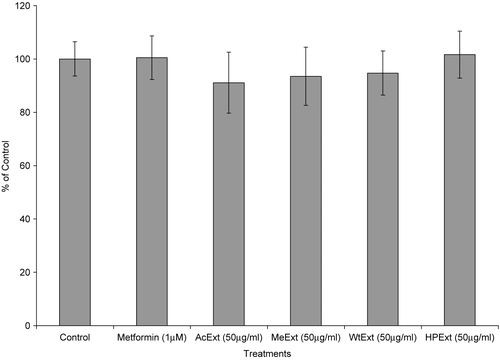
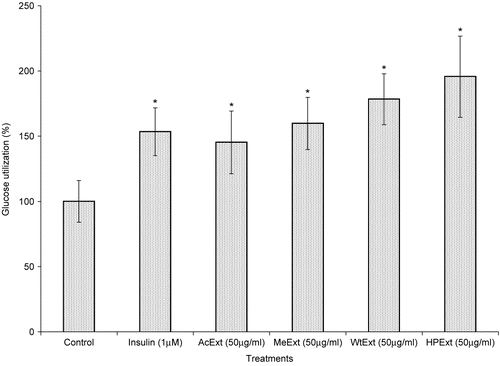
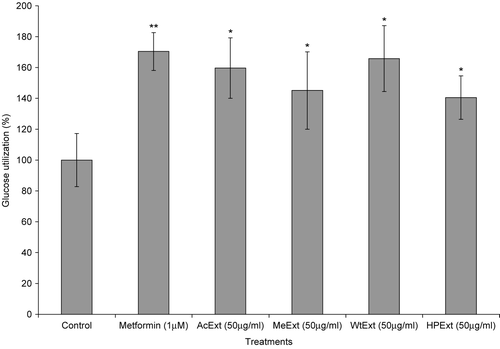
The ability of V. amygdalina extracts to increase glucose metabolism in cells was further determined using Chang-liver cell lines. The treatment of liver cells with 12.5 μg/ml of the extracts and exposed for 48 h, thereafter with 50 μg/ml of the same extracts during the glucose utilization experiment, increased glucose metabolism in the cells (p < 0.05 in all cases). The water extract was more active with a response of 165.8%, followed by acetone (159.6%), methanol (145.1%) and n-hexane/isopropanol (140.5%). However, metformin (1 μM), a standard antidiabetic drug exhibited higher activity than all the extracts with the glucose utilization response of 170.4% (p < 0.05) ().
In the MTT assay, all extracts were not toxic in the liver cells (p > 0.05 compared to control in all cases) (). The water and n-hexane/isopropanol extracts had higher responses in this assay with a relative cell number of 94.7 and 101.6% compared to 100% for the control, respectively. These observations suggest that the extracts have a high ability of stimulating glucose utilization and are not toxic in the liver cells.
Figure 4. The effect of V. amygdalina extracts on glucose utilization in the Chang-liver cells. Cells were exposed to 12.5 μg/ml extract for 48 h prior to and 50 μg/ml during the glucose utilization experiment. Data represents the mean ± SD (n = 10). AcExt: acetone extract; MeExt: methanol extract; WtExt: water extract; HPExt: n-hexane/isopropanol extract. *p < 0.05; **p < 0.005 compared to control.
Discussion
These results have established that V. amygdalina extracts increased glucose utilization in all cell lines except in the 3T3-L1 cells where the extracts lacked efficacy. In traditional medicine, the infusions made from the leaves of V. amygdalina are prepared using water, and then taken for some time until the patient experiences some recovery (CitationErasto et al., 2005). This ethnomedical information is strongly supported by the findings of this study, where the water extract demonstrated high activity in both C2C12 and Chang-liver cells, with no toxicity in the liver cells.
The ability of V. amygdalina extracts to stimulate glucose metabolism in C2C12 muscle cells highlights the possible therapy of insulin resistance in the skeletal muscles. The most interesting activities in this aspect were those of water and n-hexane/isopropanol extracts. The latter was the most active in the C2C12 assay and not toxic in the liver cells ( and ). The water extract, which was the most active in the Chang-liver cells and second in the C2C12 assay, has provided some basis for using the plant in the preparation of herbal formulations for diabetic patients.
Generally, the leaves of V. amygdalina are rich in steroidal saponins, sesquiterpene lactones and flavonoids (CitationOhigashi et al. 1991; CitationJisaka et al. 1992; CitationIgile et al. 1994; CitationErasto et al. 2006). These natural products may be responsible for the antidiabetic properties of the leaves of this plant. However, further investigation on the effect of extracts and pure compounds on the specific enzymes responsible for glucose metabolism in the skeletal muscle and liver cells is needed for better understanding of the antidiabetic property of this species. In conclusion, V. amygdalina is a potent antidiabetic plant, hence the people who have long been using its leaves for the treatment of diabetes, have possibly been relieved through the ability of its extracts to stimulate glucose metabolism in the body. Therefore, the results of this study have validated the use of V. amygdalina for the treatment of diabetes.
Acknowledgments
The authors wish to thank the National Research Foundation for financial support.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Akah PA, Ekekwe RK (1995): Ethnopharmacology of some Asteraceae family used in Nigerian traditional medicine. Fitoterapia 66: 351–355.
- Anaga AO, Njoku CJ, Ekejiuba ES, Esiaka MN, Asuzu IU (2004): Investigation of the methanolic leaf extract of Costus afer. Ker for pharmacological activities in vivo and in vitro. Phytomedicine 11: 242–248.
- Bailey CJ, Day C (2004): Avandamet: Combined metformin-rosiglitazone treatment for insulin resistance in type 2 diabetes. Int J Clin Pract 58: 867–876.
- Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, Trajanoski Z, Inzucchi S, Dresner A, Rothman DL, Shulman GI (1999): Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. New Engl J Med 341: 240–245.
- Erasto P, Adebola PO, Grierson DS, Afolayan AJ (2005): An ethnobotanical study of the plants used for the treatment of diabetes in the Eastern Cape Province, South Africa. Afr J Biotech 4:1458–1460.
- Erasto P, Grierson DS, Afolayan AJ (2006): Bioactive sesquiterpene lactones from the leaves of Vernonia amygdalina. J Ethnopharmacol 106:117–120.
- Huffman MA, Page JE, Sukhdeo MVK, Gotoh S, Kalunde MS, Towers GHN (1996): Leaf swallowing by chimpanzees: A behavioral adaptation for the control of strong nematode infections. Int J Primatol 72: 475–503.
- Igile GO, Oleszek W, Jurzysta M, Burda S, Fanfunso M, Fasanmade AA (1994): Flavonoids from Vernonia amygdalina and their antioxidant activities. J Agric Food Chem 42: 2445–2448.
- Jisaka M, Ohigashi H, Irie R, Huffman MA, Nishida T, Kaji M, Koshimizu K (1992): Bitter steroidal glucosides, vernonioside A1, A2 & A3, and their related B1 from a possible medicinal plant Vernonia amygdalina, used by wild chimpanzees. Tetrahedron 48:625–632.
- Li WL, Zheng HC, Bukuru J, De Kimpe N (2004): Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol 92: 1–21.
- Maiti R, Jana D, Das UK, Ghosh D (2004): Antidiabetic effect of aqueous extract of seed of Tamarindus indica in streptozotocin- induced diabetic rats. J Ethnopharmacol 92: 85–91.
- Marles RJ, and Farnsworth NR (1995): Antidiabetic plants and their active constituents. Phytomedicine 2: 137–189.
- Mosmann T (1983): Rapid colorimetric assay for cellular growth and survival: Application to prolifereation and cytotoxic assays. J Immunol Methods 65: 55–63.
- Ohigashi H, Jisaka M, Takagaki T, Tada T, Huffman MA, Nishida T, Kaji M, Koshimizu K (1991): Bitter principle and a related steroid glucoside from Vernonia amygdalina, a possible medicinal plant for wild chimpanzees. Agric Biol Chem 55: 1201–1203.
- Schmitz-Peiffer C, Craig DL, Biden TJ (1999): Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem 274: 24202–24210.
- Uhegbu FO (2004): Effect of aqueous extract (crude) of leaves of Vernonia amygdalina (Del.) on blood glucose, serum albumin and cholesterol levels in diabetic albino rats. Global J Pure Appl Science 10: 189–194.
- Van de Venter M, Wilson G, Roux S (2006): An optimized method to screen for in vitro antidiabetic activity. Proceedings of the Ninth International Conference on Ethnopharmacology, Nanjing, China, 22–26 August.